Abstract
Purpose
Dietary protein, as important macronutrient, is vital for intestinal function and health status. We aimed to determine the effects of dietary protein source on growth performance and intestinal function of neonates with intrauterine growth retardation (IUGR) in a pig model.
Methods
Eighteen pairs of IUGR and normal birth weight (NBW) weaned pigs were allotted to be fed starter diet containing soybean protein concentrate (SPC) or spray-dried porcine plasma (SDPP) for 2 weeks. Growth performance, antioxidant variables, intestinal morphology and absorption capability, microbiota composition and short-chain fatty acids (SCFA) were assessed.
Results
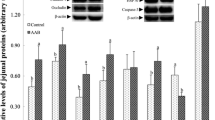
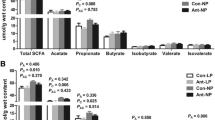
IUGR led to poor growth performance, absorption capability and changes on antioxidant variables, while SDPP diet improved the growth performance, diarrhea index, intestinal morphology and antioxidant variables of IUGR or NBW pigs relative to that fed SPC diet. Importantly, SDPP diet improved bacterial diversity and increased the abundance of phylum Firmicutes, but decreased the phylum Proteobacteria in colonic digesta, associating with higher genera Lactobacillus and lower genera Escherichia–Shigella, linking to the increased concentration of SCFA.
Conclusions
Our findings indicate that IUGR impairs the growth rate, intestinal function and oxidative status of weaned pigs, which could be partly improved by SDPP diet either for IUGR or NBW pigs, associating with the better antioxidant capability, composition of microbiotas and their metabolites.









Similar content being viewed by others
References
Wu G, Bazer FW, Wallace JM, Spencer TE (2006) Board-invited review: intrauterine growth retardation: implications for the animal sciences. J Anim Sci 84(9):2316–2337. https://doi.org/10.2527/jas.2006-156
McMillen IC, Robinson JS (2005) Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 85(2):571–633. https://doi.org/10.1152/physrev.00053.2003
Monaghan P (2008) Early growth conditions, phenotypic development and environmental change. Philos Trans R Soc Lond B Biol Sci 363(1497):1635–1645. https://doi.org/10.1098/rstb.2007.0011
Hu L, Liu Y, Yan C, Peng X, Xu Q, Xuan Y, Han F, Tian G, Fang Z, Lin Y, Xu S, Zhang K, Chen D, Wu D, Che L (2015) Postnatal nutritional restriction affects growth and immune function of piglets with intra-uterine growth restriction. Br J Nutr 114(1):53–62. https://doi.org/10.1017/S0007114515001579
Zhong X, Wang T, Zhang X, Li W (2010) Heat shock protein 70 is upregulated in the intestine of intrauterine growth retardation piglets. Cell Stress Chaperones 15(3):335–342. https://doi.org/10.1007/s12192-009-0148-3
Wang J, Chen L, Li D, Yin Y, Wang X, Li P, Dangott LJ, Hu W, Wu G (2008) Intrauterine growth restriction affects the proteomes of the small intestine, liver, and skeletal muscle in newborn pigs. J Nutr 138(1):60–66
Wang T, Huo YJ, Shi F, Xu RJ, Hutz RJ (2005) Effects of intrauterine growth retardation on development of the gastrointestinal tract in neonatal pigs. Biol Neonatol 88(1):66–72. https://doi.org/10.1159/000084645
Longo S, Bollani L, Decembrino L, Di Comite A, Angelini M, Stronati M (2013) Short-term and long-term sequelae in intrauterine growth retardation (IUGR). J Matern Fetal Neonatal Med 26(3):222–225. https://doi.org/10.3109/14767058.2012.715006
Michiels J, De Vos M, Missotten J, Ovyn A, De Smet S, Van Ginneken C (2013) Maturation of digestive function is retarded and plasma antioxidant capacity lowered in fully weaned low birth weight piglets. Br J Nutr 109(1):65–75. https://doi.org/10.1017/S0007114512000670
Biri A, Bozkurt N, Turp A, Kavutcu M, Himmetoglu O, Durak I (2007) Role of oxidative stress in intrauterine growth restriction. Gynecol Obstet Investig 64(4):187–192. https://doi.org/10.1159/000106488
Halkjaer J, Olsen A, Bjerregaard LJ, Deharveng G, Tjonneland A, Welch AA, Crowe FL, Wirfalt E, Hellstrom V, Niravong M, Touvier M, Linseisen J, Steffen A, Ocke MC, Peeters PH, Chirlaque MD, Larranaga N, Ferrari P, Contiero P, Frasca G, Engeset D, Lund E, Misirli G, Kosti M, Riboli E, Slimani N, Bingham S (2009) Intake of total, animal and plant proteins, and their food sources in 10 countries in the European Prospective Investigation into cancer and nutrition. Eur J Clin Nutr 63(Suppl 4):S16–S36. https://doi.org/10.1038/ejcn.2009.73
Boudry G, Rome V, Perrier C, Jamin A, Savary G, Le Huerou-Luron I (2014) A high-protein formula increases colonic peptide transporter 1 activity during neonatal life in low-birth-weight piglets and disturbs barrier function later in life. Br J Nutr 112(7):1073–1080. https://doi.org/10.1017/S0007114514001901
McAllan L, Skuse P, Cotter PD, O’Connor P, Cryan JF, Ross RP, Fitzgerald G, Roche HM, Nilaweera KN (2014) Protein quality and the protein to carbohydrate ratio within a high fat diet influences energy balance and the gut microbiota in C57BL/6J mice. PLoS One 9(2):e88904. https://doi.org/10.1371/journal.pone.0088904
Liu X, Blouin JM, Santacruz A, Lan A, Andriamihaja M, Wilkanowicz S, Benetti PH, Tome D, Sanz Y, Blachier F, Davila AM (2014) High-protein diet modifies colonic microbiota and luminal environment but not colonocyte metabolism in the rat model: the increased luminal bulk connection. Am J Physiol Gastrointest Liver Physiol 307(4):G459–G470. https://doi.org/10.1152/ajpgi.00400.2013
Fanca-Berthon P, Hoebler C, Mouzet E, David A, Michel C (2010) Intrauterine growth restriction not only modifies the cecocolonic microbiota in neonatal rats but also affects its activity in young adult rats. J Pediatr Gastroenterol Nutr 51(4):402–413. https://doi.org/10.1097/MPG.0b013e3181d75d52
D’Inca R, Kloareg M, Gras-Le Guen C, Le Huerou-Luron I (2010) Intrauterine growth restriction modifies the developmental pattern of intestinal structure, transcriptomic profile, and bacterial colonization in neonatal pigs. J Nutr 140(5):925–931. https://doi.org/10.3945/jn.109.116822
Hu L, Peng X, Chen H, Yan C, Liu Y, Xu Q, Fang Z, Lin Y, Xu S, Feng B, Li J, Wu D, Che L (2017) Effects of intrauterine growth retardation and Bacillus subtilis PB6 supplementation on growth performance, intestinal development and immune function of piglets during the suckling period. Eur J Nutr 56(4):1753–1765. https://doi.org/10.1007/s00394-016-1223-z
Che L, Hu L, Liu Y, Yan C, Peng X, Xu Q, Wang R, Cheng Y, Chen H, Fang Z, Lin Y, Xu S, Feng B, Chen D, Wu D (2016) Dietary nucleotides supplementation improves the intestinal development and immune function of neonates with intra-uterine growth restriction in a pig model. PLoS One 11(6):e0157314. https://doi.org/10.1371/journal.pone.0157314
Lin Y, Bolca S, Vandevijvere S, De Vriese S, Mouratidou T, De Neve M, Polet A, Van Oyen H, Van Camp J, De Backer G, De Henauw S, Huybrechts I (2011) Plant and animal protein intake and its association with overweight and obesity among the Belgian population. Br J Nutr 105(7):1106–1116. https://doi.org/10.1017/S0007114510004642
Richter CK, Skulas-Ray AC, Champagne CM, Kris-Etherton PM (2015) Plant protein and animal proteins: do they differentially affect cardiovascular disease risk? Adv Nutr 6(6):712–728. https://doi.org/10.3945/an.115.009654
Zhu Y, Lin X, Zhao F, Shi X, Li H, Li Y, Zhu W, Xu X, Li C, Zhou G (2015) Meat, dairy and plant proteins alter bacterial composition of rat gut bacteria. Sci Rep 5:15220. https://doi.org/10.1038/srep15220
Sangild PT (2006) Gut responses to enteral nutrition in preterm infants and animals. Exp Biol Med (Maywood) 231(11):1695–1711
Ferenc K, Pietrzak P, Godlewski MM, Piwowarski J, Kilianczyk R, Guilloteau P, Zabielski R (2014) Intrauterine growth retarded piglet as a model for humans—studies on the perinatal development of the gut structure and function. Reprod Biol 14(1):51–60
Su W, Zhang H, Ying Z, Li Y, Zhou L, Wang F, Zhang L, Wang T (2017) Effects of dietary l-methionine supplementation on intestinal integrity and oxidative status in intrauterine growth-retarded weanling piglets. Eur J Nutr. https://doi.org/10.1007/s00394-017-1539-3
Peace RM, Campbell J, Polo J, Crenshaw J, Russell L, Moeser A (2011) Spray-dried porcine plasma influences intestinal barrier function, inflammation, and diarrhea in weaned pigs. J Nutr 141(7):1312–1317. https://doi.org/10.3945/jn.110.136796
Che L, Zhan L, Fang Z, Lin Y, Yan T, Wu D (2012) Effects of dietary protein sources on growth performance and immune response of weanling pigs. Livest Sci 148(1–2):1–9. https://doi.org/10.1016/j.livsci.2012.04.019
Han F, Hu L, Xuan Y, Ding X, Luo Y, Bai S, He S, Zhang K, Che L (2013) Effects of high nutrient intake on the growth performance, intestinal morphology and immune function of neonatal intra-uterine growth-retarded pigs. Br J Nutr 110(10):1819–1827. https://doi.org/10.1017/S0007114513001232
Che L, Thymann T, Bering SB, I LEH-L, D’Inca R, Zhang K, Sangild PT (2010) IUGR does not predispose to necrotizing enterocolitis or compromise postnatal intestinal adaptation in preterm pigs. Pediatr Res 67(1):54–59. https://doi.org/10.1203/PDR.0b013e3181c1b15e
Owusu-Asiedu A, Baidoot SK, Nyachoti CM, Marquardt RR (2002) Response of early-weaned pigs to spray-dried porcine or animal plasma-based diets supplemented with egg-yolk antibodies against enterotoxigenic Escherichia coli. J Anim Sci 80(11):2895–2903
Kelly D, O’Brien JJ, McCracken KJ (1990) Effect of creep feeding on the incidence, duration and severity of post-weaning diarrhoea in pigs. Res Vet Sci 49(2):223–228
Mansoori B, Nodeh H, Modirsanei M, Rahbari S, Aparnak P (2009) d-Xylose absorption test: a tool for the assessment of the effect of anticoccidials on the intestinal absorptive capacity of broilers during experimental coccidiosis. Anim Feed Sci Technol 148(2–4):301–308. https://doi.org/10.1016/j.anifeedsci.2008.04.009
Zhou P, Zhao Y, Zhang P, Li Y, Gui T, Wang J, Jin C, Che L, Li J, Lin Y, Xu S, Feng B, Fang Z, Wu D (2017) Microbial mechanistic insight into the role of inulin in improving maternal health in a pregnant sow model. Front Microbiol 8:2242. https://doi.org/10.3389/fmicb.2017.02242
Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31(9):814–821. https://doi.org/10.1038/nbt.2676
McDonald D, Clemente JC, Kuczynski J, Rideout JR, Stombaugh J, Wendel D, Wilke A, Huse S, Hufnagle J, Meyer F, Knight R, Caporaso JG (2012) The biological observation matrix (BIOM) format or: how I learned to stop worrying and love the ome-ome. Gigascience 1(1):7. https://doi.org/10.1186/2047-217X-1-7
Rosero DS, Odle J, Moeser AJ, Boyd RD, van Heugten E (2015) Peroxidised dietary lipids impair intestinal function and morphology of the small intestine villi of nursery pigs in a dose-dependent manner. Br J Nutr 114(12):1985–1992. https://doi.org/10.1017/S000711451500392X
Cicalese L, Corsello T, Stevenson HL, Damiano G, Tuveri M, Zorzi D, Montalbano M, Shirafkan A, Rastellini C (2015) Evidence of absorptive function in vivo in a neo-formed bio-artificial intestinal segment using a rodent model. J Gastrointest Surg. https://doi.org/10.1007/s11605-015-2974-1
Chen J, Kang B, Jiang Q, Han M, Zhao Y, Long L, Fu C, Yao K (2018) Alpha-ketoglutarate in low-protein diets for growing pigs: effects on cecal microbial communities and parameters of microbial metabolism. Front Microbiol 9:1057. https://doi.org/10.3389/fmicb.2018.01057
Greenwood PL, Hunt AS, Bell AW (2004) Effects of birth weight and postnatal nutrition on neonatal sheep: IV. Organ growth. J Anim Sci 82(2):422–428
Beaulieu AD, Aalhus JL, Williams NH, Patience JF (2010) Impact of piglet birth weight, birth order, and litter size on subsequent growth performance, carcass quality, muscle composition, and eating quality of pork. J Anim Sci 88(8):2767–2778. https://doi.org/10.2527/jas.2009-2222
Pierce JL, Cromwell GL, Lindemann MD, Russell LE, Weaver EM (2005) Effects of spray-dried animal plasma and immunoglobulins on performance of early weaned pigs. J Anim Sci 83(12):2876–2885. https://doi.org/10.2527/2005.83122876x
Caine WR, Sauer WC, Tamminga S, Verstegen MW, Schulze H (1997) Apparent ileal digestibilities of amino acids in newly weaned pigs fed diets with protease-treated soybean meal. J Anim Sci 75(11):2962–2969
Perez-Bosque A, Miro L, Polo J, Russell L, Campbell J, Weaver E, Crenshaw J, Moreto M (2010) Dietary plasma protein supplements prevent the release of mucosal proinflammatory mediators in intestinal inflammation in rats. J Nutr 140(1):25–30. https://doi.org/10.3945/jn.109.112466
Hedegaard CJ, Strube ML, Hansen MB, Lindved BK, Lihme A, Boye M, Heegaard PM (2016) Natural Pig plasma immunoglobulins have anti-bacterial effects: potential for use as feed supplement for treatment of intestinal infections in pigs. PLoS One 11(1):e0147373. https://doi.org/10.1371/journal.pone.0147373
Jiang R, Chang X, Stoll B, Fan MZ, Arthington J, Weaver E, Campbell J, Burrin DG (2000) Dietary plasma protein reduces small intestinal growth and lamina propria cell density in early weaned pigs. J Nutr 130(1):21–26. https://doi.org/10.1093/jn/130.1.21
Pluske JR, Kim J-C, Hansen CF, Mullan BP, Payne HG, Hampson DJ, Callesen J, Wilson RH (2007) Piglet growth before and after weaning in relation to a qualitative estimate of solid (creep) feed intake during lactation: a pilot study*. Arch Anim Nutr 61(6):469–480. https://doi.org/10.1080/17450390701664249
Dong L, Zhong X, He J, Zhang L, Bai K, Xu W, Wang T, Huang X (2015) Supplementation of tributyrin improves the growth and intestinal digestive and barrier functions in intrauterine growth-restricted piglets. Clin Nutr. https://doi.org/10.1016/j.clnu.2015.03.002
Che L, Xuan Y, Hu L, Liu Y, Xu Q, Fang Z, Lin Y, Xu S, Wu D, Zhang K, Chen D (2015) Effect of postnatal nutrition restriction on the oxidative status of neonates with intrauterine growth restriction in a pig model. Neonatology 107(2):93–99. https://doi.org/10.1159/000368179
Hu L, Peng X, Qin L, Wang R, Fang Z, Lin Y, Xu S, Feng B, Wu D, Che L (2018) Dietary nucleotides supplementation during the suckling period improves the antioxidative ability of neonates with intrauterine growth retardation when using a pig model. RSC Adv 8(29):16152–16160. https://doi.org/10.1039/c8ra00701b
Yara S, Lavoie JC, Levy E (2015) Oxidative stress and DNA methylation regulation in the metabolic syndrome. Epigenomics 7(2):283–300. https://doi.org/10.2217/epi.14.84
Fang YZ, Yang S, Wu G (2002) Free radicals, antioxidants, and nutrition. Nutrition 18(10):872–879
El-Sheikh NM, Khalil FA (2011) L-arginine and l-glutamine as immunonutrients and modulating agents for oxidative stress and toxicity induced by sodium nitrite in rats. Food Chem Toxicol 49(4):758–762. https://doi.org/10.1016/j.fct.2010.11.039
Li H, Wan H, Mercier Y, Zhang X, Wu C, Wu X, Tang L, Che L, Lin Y, Xu S, Tian G, Wu D, Fang Z (2014) Changes in plasma amino acid profiles, growth performance and intestinal antioxidant capacity of piglets following increased consumption of methionine as its hydroxy analogue. Br J Nutr 112(6):855–867. https://doi.org/10.1017/S000711451400172X
Wang W, Degroote J, Van Ginneken C, Van Poucke M, Vergauwen H, Dam TM, Vanrompay D, Peelman LJ, De Smet S, Michiels J (2016) Intrauterine growth restriction in neonatal piglets affects small intestinal mucosal permeability and mRNA expression of redox-sensitive genes. FASEB J 30(2):863–873. https://doi.org/10.1096/fj.15-274779
Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH (2013) The influence of diet on the gut microbiota. Pharmacol Res 69(1):52–60. https://doi.org/10.1016/j.phrs.2012.10.020
Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P, Roca J, Dore J (2006) Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 55(2):205–211. https://doi.org/10.1136/gut.2005.073817
Li P, Niu Q, Wei Q, Zhang Y, Ma X, Kim SW, Lin M, Huang R (2017) Microbial shifts in the porcine distal gut in response to diets supplemented with Enterococcus faecalis as alternatives to antibiotics. Sci Rep 7:41395. https://doi.org/10.1038/srep41395
Kim HB, Borewicz K, White BA, Singer RS, Sreevatsan S, Tu ZJ, Isaacson RE (2012) Microbial shifts in the swine distal gut in response to the treatment with antimicrobial growth promoter, tylosin. Proc Natl Acad Sci USA 109(38):15485–15490. https://doi.org/10.1073/pnas.1205147109
Xie Z, Hu L, Li Y, Geng S, Cheng S, Fu X, Zhao S, Han X (2017) Changes of gut microbiota structure and morphology in weaned piglets treated with fresh fermented soybean meal. World J Microbiol Biotechnol 33(12):213. https://doi.org/10.1007/s11274-017-2374-7
Ley RE, Turnbaugh PJ, Klein S, Gordon JI (2006) Microbial ecology: human gut microbes associated with obesity. Nature 444(7122):1022–1023. https://doi.org/10.1038/4441022a
Mukhopadhya I, Hansen R, El-Omar EM, Hold GL (2012) IBD—what role do Proteobacteria play? Nat Rev Gastroenterol Hepatol 9(4):219–230. https://doi.org/10.1038/nrgastro.2012.14
Chen L, Xu Y, Chen X, Fang C, Zhao L, Chen F (2017) The maturing development of gut microbiota in commercial piglets during the weaning transition. Front Microbiol 8:1688. https://doi.org/10.3389/fmicb.2017.01688
Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Bjorck I, Backhed F (2015) Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of prevotella. Cell Metab 22(6):971–982. https://doi.org/10.1016/j.cmet.2015.10.001
Molbak L, Thomsen LE, Jensen TK, Bach Knudsen KE, Boye M (2007) Increased amount of Bifidobacterium thermacidophilum and Megasphaera elsdenii in the colonic microbiota of pigs fed a swine dysentery preventive diet containing chicory roots and sweet lupine. J Appl Microbiol 103(5):1853–1867. https://doi.org/10.1111/j.1365-2672.2007.03430.x
Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L (2014) The role of short-chain fatty acids in health and disease. Adv Immunol 121:91–119. https://doi.org/10.1016/B978-0-12-800100-4.00003-9
Correa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MA (2016) Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunol 5(4):e73. https://doi.org/10.1038/cti.2016.17
Kim CH, Park J, Kim M (2014) Gut microbiota-derived short-chain Fatty acids, T cells, and inflammation. Immune Netw 14(6):277–288. https://doi.org/10.4110/in.2014.14.6.277
Castillo M, Martin-Orue SM, Nofrarias M, Manzanilla EG, Gasa J (2007) Changes in caecal microbiota and mucosal morphology of weaned pigs. Vet Microbiol 124(3–4):239–247. https://doi.org/10.1016/j.vetmic.2007.04.026
Zhang C, Zhang M, Wang S, Han R, Cao Y, Hua W, Mao Y, Zhang X, Pang X, Wei C, Zhao G, Chen Y, Zhao L (2010) Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J 4(2):232–241. https://doi.org/10.1038/ismej.2009.112
Xu J, Xu C, Chen X, Cai X, Yang S, Sheng Y, Wang T (2014) Regulation of an antioxidant blend on intestinal redox status and major microbiota in early weaned piglets. Nutrition 30(5):584–589. https://doi.org/10.1016/j.nut.2013.10.018
Masanta WO, Heimesaat MM, Bereswill S, Tareen AM, Lugert R, Gross U, Zautner AE (2013) Modification of intestinal microbiota and its consequences for innate immune response in the pathogenesis of campylobacteriosis. Clin Dev Immunol 2013:526860. https://doi.org/10.1155/2013/526860
Schierack P, Walk N, Reiter K, Weyrauch KD, Wieler LH (2007) Composition of intestinal Enterobacteriaceae populations of healthy domestic pigs. Microbiology 153(Pt 11):3830–3837. https://doi.org/10.1099/mic.0.2007/010173-0
Windey K, De Preter V, Verbeke K (2012) Relevance of protein fermentation to gut health. Mol Nutr Food Res 56(1):184–196. https://doi.org/10.1002/mnfr.201100542
Yang H, Huang X, Fang S, He M, Zhao Y, Wu Z, Yang M, Zhang Z, Chen C, Huang L (2017) Unraveling the fecal microbiota and metagenomic functional capacity associated with feed efficiency in pigs. Front Microbiol 8:1555. https://doi.org/10.3389/fmicb.2017.01555
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31101727); the International Cooperation in Science and Technology Project of Sichuan Province (2014HH0034); and the Natural Science Foundation of Sichuan Province (12ZA110).
Author information
Authors and Affiliations
Contributions
The authors’ responsibilities were as follows: LQC, LH, DW and ZFF: designed the research; LH, QZ, XP, YL, JYT and YHL: conducted the research; YL, SYX, BF and JL: analyzed the data; LH: wrote the manuscript; LQC: had primary responsibility for the final contents; and all authors: read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no completing interests.
Ethical statement
The use of animals for this research was approved by the Institutional Animal Care and Use Committee of Sichuan Agricultural University.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Che, L., Hu, L., Zhou, Q. et al. Microbial insight into dietary protein source affects intestinal function of pigs with intrauterine growth retardation. Eur J Nutr 59, 327–344 (2020). https://doi.org/10.1007/s00394-019-01910-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-019-01910-z