Abstract
Purpose
Chronic exposure to stress may represent a risk factor for developing metabolic and eating disorders, mostly driven by the overconsumption of easily accessible energy-dense palatable food, although the mechanisms involved remain still unclear. In this study, we used an ethologically oriented murine model of chronic stress caused by chronic psychosocial defeat (CPD) to investigate the effects of unrestricted access to a palatable high fat diet (HFD) on food intake, body weight, energy homeostasis, and expression of different brain neuropeptides. Our aim was to shed light on the mechanisms responsible for body weight and body composition changes due to chronic social stress.
Methods
In our model of subordinate (defeated), mice (CPD) cohabitated in constant sensory contact with dominants, being forced to interact on daily basis, and were offered ad libitum access either to an HFD or to a control diet (CD). Control mice (of the same strain as CPD mice) were housed in pairs and left unstressed in their home cage (UN). In all these mice, we evaluated body weight, different adipose depots, energy metabolism, caloric intake, and neuropeptide expression.
Results
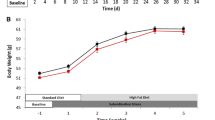
CPD mice increased the intake of HFD and reduced body weight in the presence of enhanced lipid oxidation. Resting energy expenditure and interscapular brown adipose tissue (iBAT) were increased in CPD mice, whereas epididymal adipose tissue increased only in HFD-fed unstressed mice. Propiomelanocortin mRNA levels in hypothalamic arcuate nucleus increased only in HFD-fed unstressed mice. Oxytocin mRNA levels in the paraventricular nucleus and neuropeptide Y mRNA levels within the arcuate were increased only in CD-fed CPD mice. In the arcuate, CART was increased in HFD-fed UN mice and in CD-fed CPD mice, while HFD intake suppressed CART increase in defeated animals. In the basolateral amygdala, CART expression was increased only in CPD animals on HFD.
Conclusions
CPD appears to uncouple the intake of HFD from energy homeostasis causing higher HFD intake, larger iBAT accumulation, increased energy expenditure and lipid oxidation, and lower body weight. Overall, the present study confirms the notion that the chronic activation of the stress response can be associated with metabolic disorders, altered energy homeostasis, and changes of orexigenic and anorexigenic signaling. These changes might be relevant to better understand the etiology of stress-induced obesity and eating disorders and might represent a valid therapeutic approach for the development of new therapies in this field.






Similar content being viewed by others
References
Coccurello R, D’Amato FR, Moles A (2009) Chronic social stress, hedonism and vulnerability to obesity: lessons from Rodents. Neurosci Biobehav Rev 33:537–550. doi:10.1016/j.neubiorev.2008.05.018
Berthoud H-R (2006) Homeostatic and non-homeostatic pathways involved in the control of food intake and energy balance. Obesity 14:197S–200S. doi:10.1038/oby.2006.308
Lutter M, Nestler EJ (2009) Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr 139:629–632. doi:10.3945/jn.108.097618
Volkow ND, Wise RA (2005) How can drug addiction help us understand obesity? Nat Neurosci 8:555–560. doi:10.1038/nn1452
Saper CB, Chou TC, Elmquist JK (2002) The need to feed: homeostatic and hedonic control of eating. Neuron 36:199–211. doi:10.1016/S0896-6273(02)00969-8
Björntorp P (2001) Do stress reactions cause abdominal obesity and comorbidities? Obes Rev 2:73–86. doi:10.1046/j.1467-789x.2001.00027.x
Adam TC, Epel ES (2007) Stress, eating and the reward system. Physiol Behav 91:449–458. doi:10.1016/j.physbeh.2007.04.011
Moles A, Bartolomucci A, Garbugino L et al (2006) Psychosocial stress affects energy balance in mice: modulation by social status. Psychoneuroendocrinology 31:623–633. doi:10.1016/j.psyneuen.2006.01.004
Kivimaki M, Head J, Ferrie JE et al (2006) Work stress, weight gain and weight loss: evidence for bidirectional effects of job strain on body mass index in the Whitehall II study. Int J Obes 30:982–987. doi:10.1038/sj.ijo.0803229
Dallman MF (2010) Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab 21:159–165. doi:10.1016/j.tem.2009.10.004
Martí O, Martí J, Armario A (1994) Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. Physiol Behav 55:747–753
Blanchard DC, Spencer RL, Weiss SM et al (1995) Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology 20:117–134. doi:10.1016/0306-4530(94)E0045-B
Harris RB, Zhou J, Youngblood BD et al (1998) Effect of repeated stress on body weight and body composition of rats fed low- and high-fat diets. Am J Physiol 275:R1928–R1938
Haller J, Fuchs E, Halász J, Makara GB (1999) Defeat is a major stressor in males while social instability is stressful mainly in females: towards the development of a social stress model in female rats. Brain Res Bull 50:33–39. doi:10.1016/S0361-9230(99)00087-8
Vallès A, Martí O, García A, Armario A (2000) Single exposure to stressors causes long-lasting, stress-dependent reduction of food intake in rats. Am J Physiol Regul Integr Comp Physiol 279:R1138–R1144
Fuchs E, Flügge G (2002) Social stress in tree shrews: effects on physiology, brain function, and behavior of subordinate individuals. Pharmacol Biochem Behav 73:247–258. doi:10.1016/S0091-3057(02)00795-5
Tamashiro KLK, Nguyen MMN, Sakai RR (2005) Social stress: from rodents to primates. Front Neuroendocrinol 26:27–40. doi:10.1016/j.yfrne.2005.03.001
Bartolomucci A, Pederzani T, Sacerdote P et al (2004) Behavioral and physiological characterization of male mice under chronic psychosocial stress. Psychoneuroendocrinology 29:899–910. doi:10.1016/j.psyneuen.2003.08.003
Foster MT, Solomon MB, Huhman KL, Bartness TJ (2006) Social defeat increases food intake, body mass, and adiposity in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol 290:R1284–R1293. doi:10.1152/ajpregu.00437.2005
Solomon MB, Foster MT, Bartness TJ, Huhman KL (2007) Social defeat and footshock increase body mass and adiposity in male Syrian hamsters. Am J Physiol Regul Integr Comp Physiol 292:R283–R290. doi:10.1152/ajpregu.00330.2006
Bartolomucci A, Cabassi A, Govoni P et al (2009) Metabolic consequences and vulnerability to diet-induced obesity in male mice under chronic social stress. PLoS One. doi:10.1371/journal.pone.0004331
Finger BC, Dinan TG, Cryan JF (2012) The temporal impact of chronic intermittent psychosocial stress on high-fat diet-induced alterations in body weight. Psychoneuroendocrinology 37:729–741. doi:10.1016/j.psyneuen.2011.06.015
Nonogaki K, Nozue K, Oka Y (2007) Social isolation affects the development of obesity and type 2 diabetes in mice. Endocrinology 148:4658–4666. doi:10.1210/en.2007-0296
Balsevich G, Uribe A, Wagner KV et al (2014) Interplay between diet-induced obesity and chronic stress in mice: potential role of FKBP51. J Endocrinol 222:15–26. doi:10.1530/JOE-14-0129
Sanghez V, Razzoli M, Carobbio S et al (2013) Psychosocial stress induces hyperphagia and exacerbates diet-induced insulin resistance and the manifestations of the metabolic syndrome. Psychoneuroendocrinology 38:2933–2942. doi:10.1016/j.psyneuen.2013.07.022
Blanchard RJ, McKittrick CR, Blanchard DC (2001) Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol Behav 73:261–271. doi:10.1016/S0031-9384(01)00449-8
Gaetani S, Fu J, Cassano T et al (2010) The fat-induced satiety factor oleoylethanolamide suppresses feeding through central release of oxytocin. J Neurosci 30:8096–8101. doi:10.1523/JNEUROSCI.0036-10.2010
Romano A, Potes CS, Tempesta B et al (2013) Hindbrain noradrenergic input to the hypothalamic PVN mediates the activation of oxytocinergic neurons induced by the satiety factor oleoylethanolamide. Am J Physiol Endocrinol Metab 305:E1266–E1273. doi:10.1152/ajpendo.00411.2013
Romano a., Karimian Azari E, Tempesta B et al (2014) High dietary fat intake influences the activation of specific hindbrain and hypothalamic nuclei by the satiety factor oleoylethanolamide. Physiol Behav 136:55–62. doi:10.1016/j.physbeh.2014.04.039
Miller JA (1991) The calibration of 35S or 32P with 14C-labeled brain paste or 14C-plastic standards for quantitative autoradiography using LKB Ultrofilm or Amersham Hyperfilm. Neurosci Lett 121:211–214
Paxinos G, Franklin KBJ (2004) The mouse brain in stereotaxic coordinates. Academic Press, London. doi:10.1016/S0306-4530(03)00088-X
Strissel KJ, Stancheva Z, Miyoshi H et al (2007) Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 56:2910–2918. doi:10.2337/db07-0767
Preitner F, Mody N, Graham TE et al (2009) Long-term Fenretinide treatment prevents high-fat diet-induced obesity, insulin resistance, and hepatic steatosis. Am J Physiol Endocrinol Metab 297:E1420–E1429. doi:10.1152/ajpendo.00362.2009
Mulder P, Morrison MC, Wielinga PY, et al (2015) Surgical removal of inflamed epididymal white adipose tissue attenuates the development of non-alcoholic steatohepatitis in obesity. Int J Obes (Lond):1–10. doi:10.1038/ijo.2015.226
Razzoli M, Frontini A, Gurney A, et al (2016) Stress-induced activation of brown adipose tissue prevents obesity in conditions of low adaptive thermogenesis. Mol Metab 5:19–33. doi:10.1016/j.molmet.2015.10.005
Tamashiro KLK, Nguyen MMN, Fujikawa T et al (2004) Metabolic and endocrine consequences of social stress in a visible burrow system. Physiol Behav 80:683–693. doi:10.1016/j.physbeh.2003.12.002
Choi DC, Nguyen MMN, Tamashiro KLK et al (2006) Chronic social stress in the visible burrow system modulates stress-related gene expression in the bed nucleus of the stria terminalis. Physiol Behav 89:301–310. doi:10.1016/j.physbeh.2006.05.046
Nguyen MMN, Tamashiro KLK, Melhorn SJ et al (2007) Androgenic influences on behavior, body weight, and body composition in a model of chronic social stress. Endocrinology 148:6145–6156. doi:10.1210/en.2007-0471
Lkhagvasuren B, Nakamura Y, Oka T et al (2011) Social defeat stress induces hyperthermia through activation of thermoregulatory sympathetic premotor neurons in the medullary raphe region. Eur J Neurosci 34:1442–1452. doi:10.1111/j.1460-9568.2011.07863.x
Kataoka N, Hioki H, Kaneko T, Nakamura K (2014) Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell Metab 20:346–358. doi:10.1016/j.cmet.2014.05.018
Tamashiro KLK, Hegeman M a., Sakai RR (2006) Chronic social stress in a changing dietary environment. Physiol Behav 89:536–542. doi:10.1016/j.physbeh.2006.05.026
Nishioka T, Anselmo-Franci JA, Li P et al (1998) Stress increases oxytocin release within the hypothalamic paraventricular nucleus. Brain Res 781:57–61. doi:10.1016/S0006-8993(97)01159-1
Wotjak CT, Ganster J, Kohl G et al (1998) Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience 85:1209–1222. doi:10.1016/S0306-4522(97)00683-0
Wigger A, Neumann ID (2002) Endogenous opioid regulation of stress-induced oxytocin release within the hypothalamic paraventricular nucleus is reversed in late pregnancy: a microdialysis study. Neuroscience 112:121–129. doi:10.1016/S0306-4522(02)00068-4
Raffin-Sanson ML, de Keyzer Y, Bertagna X (2003) Proopiomelanocortin, a polypeptide precursor with multiple functions: from physiology to pathological conditions. Eur J Endocrinol 149:79–90. doi:10.1530/eje.0.1490079
Pritchard LE, Turnbull AV, White A (2002) Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signalling and obesity. J Endocrinol 172:411–421. doi:10.1677/joe.0.1720411
Mountjoy KG (2015) Pro-opiomelanocortin (POMC) neurones, POMC-derived peptides, melanocortin receptors and obesity: how understanding of this system has changed over the last decade. J Neuroendocrinol 27:406–418. doi:10.1111/jne.12285
Liu S, Globa AK, Mills F et al (2016) Consumption of palatable food primes food approach behavior by rapidly increasing synaptic density in the VTA. Proc Natl Acad Sci USA 113:2520–2525. doi:10.1073/pnas.1515724113
Romano A, Cassano T, Tempesta B et al (2013) The satiety signal oleoylethanolamide stimulates oxytocin neurosecretion from rat hypothalamic neurons. Peptides 49:21–26. doi:10.1016/j.peptides.2013.08.006
Provensi G, Coccurello R, Umehara H et al (2014) Satiety factor oleoylethanolamide recruits the brain histaminergic system to inhibit food intake. Proc Natl Acad Sci USA 111:11527–11532. doi:10.1073/pnas.1322016111
Romano A, Tempesta B, Di Bonaventura MVM, Gaetani S (2016) From autism to eating disorders and more: the role of oxytocin in neuropsychiatric disorders. Front Neurosci. doi:10.3389/fnins.2015.00497
Callahan MF, Thore CR, Sundberg DK et al (1992) Excitotoxin paraventricular nucleus lesions: stress and endocrine reactivity and oxytocin mRNA levels. Brain Res 597:8–15. doi:10.1016/0006-8993(92)91499-5
Hashiguchi H, Ye SH, Morris M, Alexander N (1997) Single and repeated environmental stress: effect on plasma oxytocin, corticosterone, catecholamines, and behavior. Physiol Behav 61:731–736. doi:10.1016/S0031-9384(96)00527-6
Iványi T, Wiegant VM, de Wied D (1991) Differential effects of emotional and physical stress on the central and peripheral secretion of neurohypophysial hormones in male rats. Life Sci 48:1309–1316
Kalyani M, Hasselfeld K, Janik JM et al (2016) Effects of high-fat diet on stress response in male and female wildtype and prolactin knockout mice. PLoS One. doi:10.1371/journal.pone.0166416
Morton GJ, Thatcher BS, Reidelberger RD, et al (2012) Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. AJP Endocrinol Metab 302:E134–E144. doi:10.1152/ajpendo.00296.2011
Billings LB, Spero J a, Vollmer RR, Amico J a (2006) Oxytocin null mice ingest enhanced amounts of sweet solutions during light and dark cycles and during repeated shaker stress. Behav Brain Res 171:134–141. doi:10.1016/j.bbr.2006.03.028
Chambers AP, Woods SC (2012) The role of neuropeptide Y in energy homeostasis. Handb Exp Pharmacol:23–45. doi:10.1007/978-3-642-24716-3_2
Heilig M (2004) The NPY system in stress, anxiety and depression. Neuropeptides 38:213–224. doi:10.1016/j.npep.2004.05.002
Beck B (2006) Neuropeptide Y in normal eating and in genetic and dietary-induced obesity. Philos Trans R Soc Lond B Biol Sci 361:1159–1185. doi:10.1098/rstb.2006.1855
Reichmann F, Holzer P (2016) Neuropeptide Y: a stressful review. Neuropeptides 55:99–109. doi:10.1016/j.npep.2015.09.008
Ulrich-lai YM, Fulton S, Wilson M et al (2015) Stress exposure, food intake and emotional state. Stress 0:1–19. doi:10.3109/10253890.2015.1062981
Vicentic A, Jones DC (2007) The CART (cocaine- and amphetamine-regulated transcript) system in appetite and drug addiction. J Pharmacol Exp Ther 320:499–506. doi:10.1124/jpet.105.091512
Rogge G, Jones D, Hubert GW et al (2008) CART peptides: regulators of body weight, reward and other functions. Nat Rev Neurosci 9:747–758. doi:10.1038/nrn2806
Bharne AP, Borkar CD, Subhedar NK, Kokare DM (2015) Differential expression of CART in feeding and reward circuits in binge eating rat model. Behav Brain Res 291:219–231. doi:10.1016/j.bbr.2015.05.030
Koylu EO, Balkan B, Kuhar MJ, Pogun S (2006) Cocaine and amphetamine regulated transcript (CART) and the stress response. Peptides 27:1956–1969. doi:10.1016/j.peptides.2006.03.032
Rademacher DJ, Sullivan EM, Figge D a (2010) The effects of infusions of CART 55–102 into the basolateral amygdala on amphetamine-induced conditioned place preference in rats. Psychopharmacology (Berl) 208:499–509. doi:10.1007/s00213-009-1748-4
Ulrich-Lai YM, Christiansen AM, Ostrander MM et al (2010) Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci USA 107:20529–20534. doi:10.1073/pnas.1007740107
Mcewen BS (1998) Protectice and damaging effects of stress mediators. N Engl J Med 338:171–179. doi:10.1056/NEJM199801153380307
Dallman MF, Pecoraro N, Akana SF, et al (2003) Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci 100:11696–11701. doi:10.1073/pnas.1934666100
Dallman MF, La Fleur SE, Pecoraro NC et al (2004) Minireview: glucocorticoids—food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology 145:2633–2638. doi:10.1210/en.2004-0037
Sapolsky RM, Romero LM, Munck a. U (2000) How do glucocorticoids influence stress responses†¯? Preparative actions*. Endocr Rev 21:55–89. doi:10.1210/er.21.1.55
Dallman MF, Strack AM, Akana SF et al (1993) Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol 14:303–347. doi:10.1006/frne.1993.1010
Acknowledgements
This research was supported by the Italian Ministry for Education, University and Research, (PRIN 2009ESX7T3). The authors gratefully acknowledge the C.N.R./E.M.M.A. animal research facility (Monterotondo, Rome, Italy). All authors have read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical standards
All the experiments were conducted in accordance with Italian National Laws (DL 116/92), with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and regulations on the use of animals for research, and NIH guidelines on animal care. All the experimental and testing procedures were also in compliance with the recommendations of the European Union concerning animal welfare care and husbandry (2007/526/CE), approved by the internal ethics committee, performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments, and specifically approved by the Italian Ministry of Health by the ministerial decree N 97/2012-B.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
R. Coccurello and A. Romano equally contributed.
Rights and permissions
About this article
Cite this article
Coccurello, R., Romano, A., Giacovazzo, G. et al. Increased intake of energy-dense diet and negative energy balance in a mouse model of chronic psychosocial defeat. Eur J Nutr 57, 1485–1498 (2018). https://doi.org/10.1007/s00394-017-1434-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-017-1434-y