Abstract
Purpose
Poor vitamin B12 (B12) status is associated with adverse outcomes in pregnancy and infancy. Little is known about effects of B12 supplementation on immune function. The present study aimed to evaluate effects of pre- and postnatal B12 supplementation on biomarkers of B12 status and vaccine-specific responses in mothers and infants.
Method
In a blinded, placebo-controlled trial, Bangladeshi women (n = 68, age 18–35 years, hemoglobin <110 g/L, 11–14 weeks pregnant) were randomized to receive 250 μg/day B12 or a placebo throughout pregnancy and 3-month postpartum along with 60 mg iron + 400 μg folate. Women were immunized with pandemic influenza A (H1N1) vaccine at 26- to 28-week gestation. Blood from mothers (baseline, 72-h post-delivery, 3-month postpartum), newborns and infants (3-month) was analyzed for hemoglobin, B12, methylmalonic acid (MMA), total homocysteine (tHcy), ferritin and serum transferrin receptor, C-reactive protein (CRP) and alpha-1-acid glycoprotein (AGP). Vitamin B12 was also assessed in breast milk. H1N1-specific antibodies were determined in plasma and colostrum/breast milk.
Results
At baseline, 26 % women were B12 deficient (<150 pmol/L), 40 % had marginal status (150–220 pmol/L), 43 % had elevated MMA (>271 nmol/L), and 31 % had elevated tHcy (>10 μmol/L). Supplementation increased B12 in plasma, colostrums and breast milk (p < 0.05) and lowered MMA in neonates, mothers and infants at 3 months (p < 0.05). B12 supplementation significantly increased H1N1-specific IgA responses in plasma and colostrums in mothers and reduced proportion of infants with elevated AGP and CRP compared with placebo.
Conclusion
Supplementation with 250 μg/day B12 during pregnancy and lactation substantially improved maternal, infant and breast milk B12 status. Maternal supplementation improved H1N1 vaccine-specific responses in mothers only and may alleviate inflammatory responses in infants.
Similar content being viewed by others
Introduction
Deficiency of vitamin B12 is highly prevalent in women of reproductive age, particularly among populations with limited intake of animal source foods [1, 2]. In addition to increased requirements during pregnancy and lactation [3], another risk factor for impaired vitamin B12 status is malabsorption and diarrheal infections [4, 5]. Impaired vitamin B12 status during pregnancy is associated with increased risk of birth defects and common pregnancy complications such as intrauterine growth restriction, preterm delivery and neural tube defects [6, 7]. Reported prevalence of B12 deficiency (serum or plasma B12 <150 pmol/L) among pregnant women in South Asia is scarce [8–10]. According to the recently completed national micronutrient status survey in Bangladesh, B12 deficiency (<148 pmol/L) and marginal deficiency (<221 pmol/L) were 15.9 and 6.1 %, respectively, among non-pregnant, non-lactating women [11]. The JiVitA study in rural north-western Bangladesh found a prevalence of 20 % deficiency in early pregnancy [12]. The MINIMAT trial in Matlab reported that 46 % of women had deficient B12 status early in their third trimester [13]. In the same cohort, B12 deficiency was also present in 37 % of infants at 6 months of age [14].
Anemia is a major public health problem in Bangladeshi women and infants [14, 15]. In light of B12 deficiency, it is of critical importance to know whether inclusion of vitamin B12 in addition to iron–folate given during pregnancy and lactation under the national program in Bangladesh can improve hematologic features in mothers and infants and improve their B12 status. In spite of the high global prevalence of B12 deficiency and its serious effects on pregnant women and offspring, there are no reported supplementation trials that have used adequate amounts of B12 in pregnancy and postpartum.
There are limited data on the role of vitamin B12 on immunomodulatory effects on innate and adaptive immunity [16, 17]. Only one study reported that low serum B12 concentrations in immunocompetent elderly subjects impaired antibody response to pneumococcal polysaccharide vaccine [18]. There is a need to further explore the role of B12 on vaccine-specific adaptive immunity. The licensed inactivated influenza vaccine is recommended for pregnant women but not infants. Studies in Bangladesh demonstrated that influenza vaccination of pregnant women during the second–third trimesters can provide their infants with passive protection from respiratory illness [19, 20]. We conducted a randomized, placebo-controlled clinical trial in Bangladesh to assess the effect of B12 supplementation in pregnancy and lactation on alleviation of anemia, and improvement of B12 status and vaccine-specific immunity in mothers and infants.
Methods
Study area
The site selected was an urban maternity clinic in the Maternal and Child Health Training Institute (MCHTI) in Azimpur, Dhaka. The hospital caters to the low- and middle-income residents of Azimpur and the surrounding areas. Pregnant women living in the communities of Azimpur and Kamrangir Char were recruited through the clinic.
Selection of participants
There were two female field workers (FFW) supervised by a Field Research Supervisor (FRS) based on the office in MCHTI. The FFW initially surveyed the study field site for potential study participants placing them on a screening list. After consenting, the participants became eligible for the study once they passed the inclusion criteria. The FFW invited women aged 18–35 years at an early stage of pregnancy (11–14 weeks) to participate in this randomized clinical trial from June 2010 to August 2012. Pregnant women were identified by reported history of a missed menstrual period. The FFW explained the research study to women and asked for their willingness to deliver their infant at the MCHTI. Those who agreed to participate provided signed consent in the presence of family members (usually mother, mother-in-law or husband) as a witness. The field supervisor then scheduled clinic visits for blood drawing. Eligible participants (Hb <110 g/L to enable evaluation of the effects of B12 on anemia) were enrolled consecutively, assigned to a B12 supplemented or placebo group, and followed for about 9 months. Exclusion criteria were severe anemia (Hb <70 g/L); <2 years gap between pregnancies; history or presence of systemic disease, complicated pregnancy or preterm delivery, and during the study, abortion; and history of hypersensitivity to influenza vaccine.
Ethics
The study protocol was reviewed and approved by the Ethical Review Committee of the International Centre for Diarrheal Disease Research, Bangladesh (ICDDR,B), and the Human Research Protection Office at UC Davis.
Intervention
No toxic or adverse effects, including maternal or fetal complications, have been reported with large intakes of vitamin B12 from food or supplements [21]. Ampola et al. [22] first demonstrated that a single pharmacologic dose of 10 mg B12 supplement followed by daily intramuscular dose of 5 mg, from 34 weeks of gestation to 41 weeks after delivery, reverted methylmalonic acidemia status in the mother and the infant with no serious adverse effects. Kuzminski et al. [23] further showed that in B12 deficiency, 2 mg of cyanocobalamin administered orally on a daily basis was superior to 1 mg administered intramuscularly on a monthly basis. A pharmacological dose of B12 (250 μg/day) was chosen in this study because previous studies have shown minimal efficacy when recommended dietary allowance (RDA; 2.6 µg/day) was provided [14].
Enrolled women were randomly assigned to receive either B12 supplements or a placebo pill identical in appearance and taste. The vitamin B12 and placebo supplements were prepared, packaged, and blinded by the Incepta Pharmaceuticals Company in Bangladesh and delivered in small bottles of ten tablets. Bottles were labeled as A and B, and the identity was kept confidential until the end of the study. The tablet, either A or B, to be received by the first participant was determined by lottery, and thereafter, every alternate participant received that type of tablet. The vitamin B12 tablets were masked for taste and flavor and were indistinguishable in appearance, color and taste from the placebo tablets. The IDs of the participants were sequential. Participants and caregivers were blinded to the randomization until laboratory work and statistical analysis were completed. Both groups also received daily dose of 60 mg iron and 400 µg of folic acid (iron–folate) as part of the routine care of pregnant women delivered by the Health, Nutrition, and Population Sector Program and implemented by the Ministry of Health and Family Welfare of Bangladesh. Daily supplementation with iron–folate and vitamin B12 or placebo was continued until 3-month postpartum.
Vaccination against pandemic influenza A (H1N1)
At 26–28 weeks of gestation, all women received influenza immunization with the A/California/7/2009 (H1N1) v-like strain (X-179A) LOT: AFLSA203AA (National Institute for Biological Standards and Control, Hertfordshire, UK). A trained hospital nurse administered the vaccine intramuscularly, and participants were observed for 15 min after vaccination.
Compliance, anthropometric and dietary data collection
The FFW made weekly home visits to deliver that week’s dose of either placebo or supplements to mothers in person, and while in their home, they checked the consumption of the tablets on memory cards completed by the women and counted leftover pills. During the first visit to the clinic, maternal height was measured to the nearest 0.1 cm using a wall-mounted stadiometer (locally manufactured and calibrated), and body weight to the nearest 0.005 kg with an electronic weighing scale, after removal of shoes and heavy clothing. Dietary intake of B12- and folate-rich foods by study participants was recorded at the beginning and end of follow-up using a dietary diversity questionnaire previously validated in this community.
Sample collection
Peripheral blood (at enrollment, 72-h post-delivery and 3-month postpartum), colostrum (5 mL, within 72-h post-delivery) and breast milk (5 mL, 3-month postpartum) from mothers, and peripheral blood from infants (3-month) were collected. At birth, a cord blood (10 mL) sample from the umbilical vein was collected into Li-heparin tube (Becton–Dickinson, Franklin Lakes, NJ, USA), by a trained nurse at the clinic. Maternal (7 mL) and infant (2.5 mL) blood samples were collected by venipuncture from mothers and infants in Li-heparin-coated tubes covered with foil. Manually expressed breast milk was collected from the mothers in the clinic, usually at the end of a breastfeeding session. All samples were aliquoted and stored at −80 °C until analysis.
Biochemical analyses
The quantitative determination of total Hb in whole blood (500 μL) was made by spectrophotometry (SIGMA kit, St Louis, MO, USA) after conversion of Hb to cyanmethemoglobin. Soluble transferrin receptors (sTfR) and alpha-1-acid glycoprotein (AGP) were determined by immunoturbidimetric assay on a Roche automated clinical chemistry analyzer (Hitachi-902, Boehringer Mannheim, Germany). Plasma ferritin, folate and B12 were measured by an electrochemiluminescence immunoassay method using immunoassay analyzer (Cobas e6000, Roche Diagnostics GmbH, Mannheim, Germany) in the NBL at ICDDR,B. At the Western Human Nutrition Research Center (WHNRC) in Davis, CA, plasma MMA was analyzed by liquid chromatography–tandem mass-spectrometry (UPLC-MS/MS) [24] and plasma tHcy was determined by high-performance liquid chromatography with fluorescence detection (HPLC-FLD; Agilent 1200; Burnsville, MN, USA) [25]. Breast milk B12 analysis was carried out on a Siemens IMMULITE® automated, quantitative immunoassay analyzer (Duluth, GA, USA) at WHNRC [26]. Hs-CRP was analyzed by Cobas Integra® 400 plus Analyzer (Roche Diagnostics, Indianapolis, USA). The within- and between-assay coefficients of variation for plasma samples were <10 % for all analytes.
Vaccine-specific antibody responses
Influenza vaccine-specific antibody responses in plasma (IgA, IgG) and colostrum/breast milk (IgA) were measured by enzyme-linked immunoadsorbent assay (ELISA) at the NBL. Duplicate samples were tested, and a human serum reference standard (WHO International Standard for antibody to influenza H1N1pdm virus; NIBSC code: 09/194) was serially diluted to generate a reference curve. Briefly, plates (MaxiSorp F96 by NUNC, Naperville, IL, USA) were coated with 1 µg/mL of Influenza Antigen A/California/7/2009 (H1N1)v (NYMC-X179A) (Egg Derived) (NIBSC, UK). After an overnight incubation, ELISA plates were washed with PBS + 0.05 % Tween-20 and non-specific binding was blocked with 3 % BSA in PBS. Standards and samples were added in duplicates and incubated for an hour followed by washing and incubation with conjugates (goat antihuman IgA-HRP or mouse antihuman IgG (Fc)-HRP) for 1 h. Color was developed by adding 1-Step Ultra TMB-ELISA (Vector PK4000 Kit, Vector Laboratories, Burlingame, CA, USA), and reaction was stopped with 1 M sulfuric acid. Plate was read at 450 nm in a microplate reader (Labsystem Multiskan Ascent, Vienna, VA, USA). The concentration of influenza-specific IgG or IgA was derived by extrapolation from the standard curve generated from the reference serum (mIU/mL).
Diagnostic cutoffs
Mild anemia was defined as <110 g/L of Hb in pregnancy [27] or <105 g/L in infants [28]. Cutoffs of <150, 150–220 and >221 pmol/L were used to classify mothers and infants as having deficient, marginal or adequate plasma vitamin B12 status, respectively [29]. Folate concentrations <5 nmol/L were defined as deficient [30]. Elevated MMA was defined as >271 nmol/L [31, 32], and elevated tHcy (hyperhomocysteinemia) as >10 and >12 μmol/L during pregnancy and lactation, respectively [33]. Serum ferritin <15 and <12 μg/L was used to classify mothers and infants with low iron store, respectively [34]. sTfR >8.5 mg/L indicated iron deficiency [35]. The cutoffs of >1.2 g/L for AGP in mothers [36] and >0.6 g/L in infants [37] and >5.0 mg/L for CRP [36] in both groups have been used to indicate inflammation, where “any inflammation” was defined by elevated CRP or AGP. Immune response was defined as a twofold or greater increase in influenza vaccine-specific antibody levels at 3-month postpartum.
Sample size
Since there were no data on the effect of maternal vitamin B12 supplementation on infant B12 status, we calculated sample size based on the following study. Supplementation of HIV-infected pregnant women with multivitamins (vitamins B, C and E) containing 50 µg B12 throughout pregnancy and 6-month postpartum increased infant serum B12 concentration by 127 pmol/L with a standard deviation of 165 pmol/L [38]. Using 90 % power and a significance level of ≤0.05, the estimated sample size to detect effects of maternal supplementation on infant plasma vitamin B12 was 37/group. Considering a dropout of 10 %, the required sample size in each group was 41.
Statistical methods
Statistical analyses were conducted using SPSS (version 17.0) for WINDOWS software (SPSS, Inc., Chicago, IL). Differences were significant at p ≤ 0.05. Descriptive statistics were performed to assess normality of the distribution of the biochemical variables. Outcome variables that did not conform to a normal distribution (i.e., plasma and breast milk vitamin B12, plasma MMA, tHcy, ferritin and sTfR, vaccine-specific IgG and IgA) were log-transformed before analyses. Continuous variables were summarized as median with 25th and 75th percentiles/mean ± standard deviation/geometric mean with 95 % confidence intervals. An unpaired t test was used to assess differences in baseline characteristics between the placebo and vitamin B12 groups. Analysis of covariance (ANCOVA) compared these groups at each time point, controlling for baseline values and all significant covariates. The Chi-square test compared the percentage of sero-converted (>twofold increase in titer) participants between groups. Bivariate correlations between variables including maternal and infant plasma B12, MMA and tHcy, and colostrum and breast milk B12, vaccine-specific IgG and IgA were examined by using Pearson’s correlation coefficient (r).
Results
Recruitment and participant flow
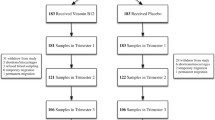
Even though the inclusion criteria were to enroll pregnant women with Hb <110 g/L, in urban slums of Dhaka city, it was difficult to find women at gestation week 11–14 with this cutoff. Many women have already started the intake of iron and folate pills as early as 8–10 weeks of pregnancy, and concentration of Hb ranged from 87 to 117 g/L among these women. A total of 82 women were enrolled in the study, of whom 41 were randomized to the placebo and 41 to the B12 supplement groups. During the study period, four participants aborted, five migrated, two refused to take the tablets or withdrew, and three were lost to follow-up (Fig. 1). Thus, the final analysis included 68 mother and infant pairs (35 in the placebo and 33 in the B12 group). The compliance of complete intake of tablets was 95 % in placebo group and 94 % in B12 group. Analysis of data was based on intention to treat.
Baseline values
Demographic and biochemical variables did not differ between groups at baseline (Table 1). The mean age of women was 22.5 ± 3.6 years, and mean BMI was 21.2 ± 4 kg/m2. About 46 % women were anemic at baseline (Table 2). There was no significant difference in the proportion of anemic women between the two groups at enrollment (placebo, 41 % vs. B12-group, 50 %, p = 0.62). Among these women, only 16 % were iron deficient defined by low ferritin and only one woman had elevated sTfR. Vitamin B12 insufficiency was quite common among these women as was the prevalence of elevated MMA and tHcy (hyperhomocysteinemia) (Table 2). A woman was considered to have impaired B12 status if abnormal concentrations were obtained for B12 and one or more additional biomarkers. It was more common to have impaired status with any two biomarkers rather than all three biomarkers (B12, MMA and tHcy) (Table 2).
A questionnaire tool was applied to collect information on consumption of animal source food (ASF), rich in B12 (meat, egg, fish and dairy products) during the previous 7 days. The consumption pattern of ASF was similar in both groups at baseline. Consumption of at least two ASF was 42 % in the supplemented and 36.4 % in the placebo group (p = 0.67), while only 2 % participants in both groups consumed all four ASF.
Associations among biomarkers at baseline
Plasma vitamin B12 was positively correlated with folate and inversely with MMA (Supplementary Fig. 1). tHcy was positively correlated with plasma MMA and inversely with plasma folate (Supplementary Fig. 2) but not with plasma B12. Baseline ferritin was strongly correlated with folate, likely due to the combined iron–folate supplementation. Hb and B12 were not correlated with each other and neither correlated with either of the markers of acute phase response (AGP and Hs-CRP). sTfR was correlated with AGP and, as expected, inversely correlated with ferritin (Supplementary Table 1).
Maternal vitamin B12 status
Supplementation significantly increased median plasma B12 in mothers (267 vs. 142 pmol/L) at 72-h post-delivery and (416 vs. 242 pmol/L) at 3-month postpartum compared with placebo group (all p < 0.001) (Fig. 2a). Only one woman in the supplemented group remained in the deficient range and one in the marginal range at 3-month postpartum. In contrast, in the placebo group, about 29 % (10/35) women remained marginally deficient and one remained in the deficient range. Supplementation significantly lowered median MMA (198 vs. 305 nmol/L, p < 0.001) at 72-h post-delivery and (191 vs. 294 nmol/L, p < 0.001) at 3-month postpartum in mothers (Fig. 2b) compared with placebo group. The prevalence of elevated MMA was 18 % (6/33) among mothers at 3 months of lactation in the supplemented group compared with 60 % (21/35) in the placebo group. However, plasma tHcy concentrations in mothers at 3-month postpartum [elevated tHcy: 12 % (4/33) for B12 group, vs. 23 % (8/35) for placebo group, p = 0.25] were not significantly affected by supplementation (Fig. 2c).
Plasma concentrations of a vitamin B12 (pmol/L), b MMA (nmol/L), c tHcy (µmol/L) at defined time points (mothers: baseline at gestation week 11–14 and 3-month postpartum; infant: at birth and 3 month) in the placebo and B12 groups. The box plot shows the median and 25th and 75th percentiles. ANCOVA was performed to compare between groups, controlling for baseline values. ★ p < 0.05; ★★ p < 0.01; ★★★ p < 0.001
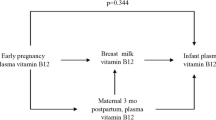
Median B12 in colostrum of the supplemented group increased greatly to 778 versus 320 pmol/L in the placebo group (p < 0.001). Also, at 3 months of lactation, the median milk B12 concentrations in the supplemented women were significantly higher compared with the placebo group (235 vs. 170 pmol/L, p = 0.03) (Fig. 3).
Infants vitamin B12 status
The majority (91 %) of infants was born at term (after 38.8 ± 1.8 weeks of gestation) (Table 1). There were four preterm births in the B12 group and two in the placebo group (born before 37 weeks of gestation). There were no significant differences in birth weight between the two groups (p = 0.87). There were only two low birth weight infants (<2,500 g) in the Placebo group, and one in the B12 group.
In the placebo group, three newborns were B12 deficient and six were marginally deficient. In the B12 group, two newborns were marginally deficient in vitamin B12; however, no overt B12 deficiency was found. Furthermore, six infants of placebo group with adequate B12 status at birth became B12 deficient after 3 months; similarly, one infant in the B12 group became B12 deficient after 3 months probably due to non-compliance of the mother. Supplementation increased median plasma B12 in newborns (555 vs. 208 pmol/L) and in infants (328 vs. 200 pmol/L) at 3 months (all p < 0.001) (Fig. 2a) compared with the placebo group. In the supplemented group, 85 % (28/33) infants had adequate B12 status. Only three infants remained in the deficient range and two in the marginal range. In contrast, only 36 % infants in the placebo group had adequate B12 status, about 29 % (10/34) remained B12 deficient, and 35 % (12/34) had marginal B12 status.
Supplementation lowered MMA in newborns (291 vs. 424 nmol/L, p = 0.003) and infants (256 vs. 351 nmol/L, p = 0.017) at 3 months (Fig. 2b) and reduced tHcy in newborns (8 vs. 11 µmol/L, p = 0.027) and infants (9 vs. 14 µmol/L, p < 0.001) (Fig. 2c).
Anemia and iron status
Vitamin B12 supplementation had no significant effects on mean Hb concentrations or on iron status biomarkers (ferritin or sTfR) of mothers or infants at 3 months (Table 3). Maternal median Hb increased in all women at 3 months most likely due to intake of iron and folate supplements (baseline vs. 3 months, 113 g/L vs. 130 g/L, p = 0.06). Prevalence of anemia decreased in both groups and was comparable in mothers (from baseline to 3-month postpartum, 41–38 % in B12 group and 50–34 % in placebo group) and infants (29 % for B12 group vs. 34 % for placebo) at 3 months. When women with baseline Hb <110 g/L were considered, there was no difference in the increase in Hb levels at 3-month postpartum between the two groups (p = 0.11).
H1N1 vaccine-specific adaptive response
Pre-immunization geometric mean titers (GMT) (IgG, IgA) for H1N1 influenza vaccine antigens were similar in the two groups at baseline. In general, the GMT of vaccine-specific IgA or IgG peaked in cord plasma and then decreased in mothers by 3-month postpartum. Interestingly, B12 supplementation significantly increased vaccine-specific plasma IgA concentrations in mothers at 3-month postpartum compared with the placebo group (p = 0.032) (Fig. 4a). However, unlike mothers, H1N1 IgA response in infants was not different between the two groups (Fig. 4a).
Plasma H1N1 vaccine-specific a IgA and b IgG antibody titers at defined time points (mothers: baseline at gestation week 11–14 and 3-month postpartum; infant: 3 month). Geometric mean titer is given as horizontal bar in each column. The left Y axis is used for mothers, and the right Y axis is used for the infants. ★ p < 0.05
At 3-month postpartum, the percentage of mothers with a twofold or greater increase in H1N1-IgG titers did not differ (p = 0.17) between groups (56 vs. 37 % for B12 and placebo groups, respectively). There was a trend for higher H1N1 IgG responses in infants of supplemented mothers compared with the infants of placebo mothers (p = 0.09) (Fig. 4b).
In both groups, the mean H1N1-specific IgA antibody titer was notably higher in the colostrum compared with the breast milk at 3-month postpartum. Colostrum in the supplemented group showed a greater IgA antibody response to the H1N1 vaccine than the placebo group (p = 0.04). However, the difference between the two groups was not significant in breast milk at 3 months of lactation (Table 4).
In the supplemented mothers, vitamin B12 in colostrum was strongly correlated with colostrum H1N1 IgA; however, this association was not significant for placebo mothers (Fig. 5a, b). Similarly, breast milk B12 was correlated with H1N1 IgA in the supplemented but not in the placebo group (Fig. 5c, d).
Maternal plasma vitamin B12 versus concentration of H1N1-specific IgA in colostrum in a B12 (r = 0.41; p = 0.02; n = 32) and b placebo (r = 0.10; p = 0.60; n = 29) groups. Maternal plasma vitamin B12 versus H1N1-specific IgA in breast milk in c B12 (r = 0.39; p = 0.04; n = 28) and d placebo (r = 0.07; p = 0.73; n = 27) groups. All values are expressed in log10 scale
Acute phase response
Concentrations of maternal plasma acute phase markers (AGP and Hs-CRP) did not differ significantly between the two groups at baseline or at 3-month postpartum. Intriguingly, plasma AGP in infants of supplemented group was significantly lower than the placebo group at 3 months (p = 0.05) (Table 4). Infants of the supplemented group tended to have lower concentrations of Hs-CRP compared with the placebo group, though this difference was not statistically significant (p = 0.11). However, the proportion of infants having Hs-CRP >5.0 mg/L (p = 0.03) or AGP >0.6 g/L (p = 0.06) in the B12 group were significantly lower compared with the placebo group (Table 4).
Discussion
In this study, we found that maternal status of plasma vitamin B12 and MMA and colostrum, and breast milk B12 is substantially improved by 250 μg/day (i.e., 96–100-fold higher than RDA) supplements throughout pregnancy and lactation. In addition, infants born to these supplemented mothers had improved vitamin B12 status, i.e., higher plasma B12 and lower plasma tHcy and MMA concentrations. B12 supplementation significantly improved maternal vaccine-specific IgA response. To our knowledge, this is the first study to show beneficial effects of maternal B12 supplementation on the vaccine-specific adaptive immunity.
In regions such as Bangladesh, many women have inadequate vitamin B12 status, most likely due to low intake of animal source food. According to the national micronutrient status survey 2011–2012, average consumption of B12 from food is 2.07 µg/day among non-pregnant, non-lactating women [11]. Our findings also suggest limited intake of animal source food during pregnancy. The current US RDA for vitamin B12 is 2.6 µg/day in pregnancy and 2.8 µg/day in lactation [39]. Fetal demand for vitamin B12 is approximately 0.3 µg/day [40]. Only a small percentage of vitamin B12 is absorbed when given orally in high doses [1]. It is thus quite likely that the 250 µg supplement/day delivered a few µg of absorbed B12 to the women; however, this dose was effective in raising B12 status of both mothers (416 pmol/L) and infants (328 pmol/L) up to 3-month postpartum. A recent study by Duggan et al. [41] showed that a dose of 50 µg/day throughout pregnancy up to 6-week postpartum among urban Indian women raised plasma B12 concentrations to 216 pmol/L in the second trimester. However, this dose did not maintain B12 concentrations above 220 pmol/L (150–220 pmol/L; marginal deficiency) in mothers during the third trimester or in the infants at 6 weeks (199 pmol/L). In the MINIMat trial, multiple micronutrient supplementation (containing 2.6 µg/day B12, RDA) from first trimester up to 3-month postpartum decreased B12 deficiency level in infants in the multiple micronutrient group by 11 %, but no effects were noted in the mothers [14]. Interestingly, when multivitamins containing 50 µg B12 were given to HIV-infected pregnant women throughout pregnancy up to 6-month postpartum, this dose in the multivitamins was sufficient to increase infant serum B12 concentration (423 pmol/L) [38]. In our study, 250 µg B12/day increased B12 concentrations in both colostrums and breast milk. Similarly, Duggan et al. demonstrated elevated concentrations of B12 in breast milk up to 6-week postpartum in the supplemented group. However, the concentrations were much lower (97 pmol/L) than those obtained in our study (235 pmol//L) [41]. These results suggest that both the dose and the duration of supplementation are important for sustaining optimum concentration of B12 in circulation. Additional randomized controlled trials are needed to test the efficacy of supplemental doses between the apparently inadequate amount when provided as the RDA [14] and the high dose provided here and the duration of supplementation.
The prevalence of impaired vitamin B12 status, elevated MMA and tHcy in early pregnancy in the present study is in agreement with that previously reported in similar B12-deficient populations in India [42] and Nepal [10]. Supplementation with 250 μg/day B12 reduced MMA and tHcy concentrations in Bangladeshi women. Corroborating our finding, a study in rural Mexico showed that a single mega dose of intramuscular injection of B12 followed by 500 μg/day orally for 3 months in non-pregnant and non-lactating women reduced MMA and tHcy in plasma [43]. In contrast, the 50 µg/day B12 dose in Indian women did not have any impact on MMA and tHcy concentrations during pregnancy. However, lowering of MMA and tHcy concentrations was evident in their infants [41] albeit higher than those observed in newborns and infants in our study. An important observation was that the achieved levels of B12 and MMA in Bangladeshi infants in the supplemented group were comparable with those of “healthy” Norwegian infants with adequate B12 status [44].
Similar to other studies [43, 45], vitamin B12 supplementation did not further improve Hb levels and iron status biomarkers of mothers at 3-month postpartum or infants. Likely, maternal Hb was increased due to concomitant supplementation of all participants with 60 mg/day iron and 400 µg/day folic acid throughout the study. Folate deficiency was absent in this study population, probably due to successful implementation of government program of folic acid supplementation throughout pregnancy and postpartum.
Recent studies show a high burden of influenza in children <6 months of age in Bangladesh [46]. A series of studies in Bangladesh demonstrated that influenza and pneumococcal vaccination of pregnant women can provide their infants with passive protection from respiratory illness via transfer of vaccine-specific antibodies through fetal/placental transfer and breast milk [19, 46–48]. Since vitamin B12 plays a critical role in DNA synthesis, the proliferation of rapidly dividing lymphoid and hematologic cells could be impaired in vitamin B12 deficiency. Earlier clinical and experimental observations have demonstrated association between B12 deficiency and hypogammaglobulinemia [49–52]. In elderly patients with pernicious anemia, cyanocobalamin (B12) treatment increased concentrations of immunoglobulins (IgG, IgA and IgM) and complements (C3) and restored cell-mediated immunity [53]. Given the fact that infant vitamin B12 deficiency is secondary to maternal deficiency [54], we hypothesized that poor B12 status may impair vaccine-specific immunity of both mothers and infants. However, we found that B12 supplementation increased post-immunization plasma H1N1-IgA in mothers only but not in children. Both groups had similar levels of vaccine-specific IgA responses in breast milk, which may partly explain the absence of intervention effects in the infants. Interestingly, in the supplemented group, H1N1-specific IgA was correlated with B12 concentration in colostrums and breast milk but not in the placebo group. Further research is warranted to elucidate whether this association is implicated in the local mucosal protection for the infant.
Transplacental transfer of IgG from mothers is important for protection of infants during the first 6 months of life when the infant immune system is not fully developed to produce IgG [55]. Earlier studies have shown a progressive decline of passive IgG antibodies in infants with a half-life of up to 35–38 days after birth [47, 48]. We found that concentration of H1N1-specific IgG was highest in the cord blood with several fold lower levels in mothers (fivefold–eightfold) and in children (20–28 fold) at 3 months. The reduction of passive H1N1-specific IgG levels in the infants appeared to be slower in the B12 group compared with the placebo group (p = 0.09). A slow disappearance of vaccine-specific IgG in the B12 group would reflect better protection against seasonal flu in infants as shown by Chu et al. [48]. A larger sample size would have more power to show a significant difference. It is also possible that the slow decline in antibody titers in the B12 group would have been more evident if measured at multiple time points, e.g., at 2, 4 and 6 months. It is noteworthy that different types of influenza viruses (influenza viruses A, B and C) circulate among the human populations. The earlier study [19] in pregnant women used trivalent inactivated influenza virus vaccine, which consists of three antigens, two from influenza A (H3N2, H1N1) virus and one from influenza B virus, while the present study used the monovalent vaccine (H1N1). This may have restricted our study findings with regards to other subtypes.
The acute phase response is a generalized reaction of the body to inflammation. Elevated acute phase proteins, AGP and CRP may be indicative of ongoing inflammatory processes. A notable finding of this study is that maternal vitamin B12 supplementation had an impact in reducing the percentage of children with elevated acute phase response. Prendergast et al. [56] reported that persistently high levels of plasma CRP and AGP in Zimbabwean infants at various intervals after birth resulted from low-grade chronic inflammation and were associated with stunting at 18 months of age. The beneficial impact of maternal B12 supplementation may also have consequences in later life health outcomes.
There were a number of limitations in the study. One drawback is the small sample size that limits statistical power to detect differences. Several factors (e.g., nutritional status of mother–infant pairs, South Asian study setting) may limit the generalizability of our study findings to other regions. Larger randomized clinical trials in other geographical locations are required to confirm these findings. Quantitative intake of food and 24-h dietary data were not collected to allow determination of B12 content in the daily diet, thereby ruling out the scope for assessing dietary B12 influence on study outcomes.
The national program in Bangladesh recommends that all pregnant and lactating women up to 3-month postpartum should receive iron–folate/day with the aim to reduce maternal anemia. Due to inadequate data, the possible significance of vitamin B12 status in pregnancy is little appreciated by policy makers. The current study provides evidence of beneficial effects of pre- and postnatal B12 supplementation on both mothers and infants. Further mechanistic studies would be of much interest to assess the impact of B12 supplementation during pregnancy and postpartum on functional measures of cellular and humoral immune responses. Future studies may explore other options, for example, by fortifying staple food—wheat flour or rice, milk or milk products (e.g., yogurt, cheese and sweetmeat) to improve maternal and subsequently infant vitamin B12 status.
Abbreviations
- AGP:
-
Alpha 1-acid glycoprotein
- GMT:
-
Geometric mean titers
- Hb:
-
Hemoglobin
- Hs-CRP:
-
High-sensitivity C-reactive protein
- ICDDR,B:
-
International Centre for Diarrheal Disease Research, Bangladesh
- MCHTI:
-
Maternal and Child Health Training Institute
- MMA:
-
Methylmalonic acid
- NBL:
-
Nutritional Biochemistry Laboratory
- RDA:
-
Recommended dietary
- tHcy:
-
Total plasma homocysteine
- WHNRC:
-
Western Human Nutrition Research Center allowance
- sTfR:
-
Soluble transferrin receptor
References
Allen LH (2009) How common is vitamin B-12 deficiency? Am J Clin Nutr 89:693S–696S
Stabler SP, Allen RH (2004) Vitamin B12 deficiency as a worldwide problem. Annu Rev Nutr 24:299–326
Allen LH (1994) Vitamin B12 metabolism and status during pregnancy, lactation and infancy. Adv Expl Med Biol 352:173–186
Casterline JE, Allen LH, Ruel MT (1997) Vitamin B-12 deficiency is very prevalent in lactating Guatemalan women and their infants at three months postpartum. Nutr 127:1966–1972
Scatliff C, Koski K, Scott M (2011) Diarrhea and novel dietary factors emerge as predictors of serum vitamin B12 in Panamanian children. Food Nutr Bull 32:54–59
Molloy AM, Kirke PN, Troendle JF, Burke H, Sutton M, Brody LC, Scott JM, Mills JL (2009) Maternal vitamin B12 status and risk of neural tube defects in a population with high neural tube defect prevalence and no folic acid fortification. Pediatrics 123:917–923
Refsum H (2001) Folate, vitamin B12 and homocysteine in relation to birth defects and pregnancy outcome. Br J Nutr 85:S109–S113
Pathak P, Kapil U, Yajnik C, Kapoor S, Dwivedi S, Singh R (2007) Iron, folate, and vitamin B12 stores among pregnant women in a rural area of Haryana State, India. Food Nutr Bull 28:435–438
Samuel TM, Duggan C, Thomas T, Bosch R, Rajendran R, Virtanen SM, Srinivasan K, Kurpad AV (2013) Vitamin B12 intake and status in early pregnancy among urban South Indian women. Ann Nutr Metab 62:113–122
Stewart C, Christian P, Schulze K, Arguello M, LeClerq S, Khatry S, West K (2011) Low maternal vitamin B-12 status is associated with offspring insulin resistance regardless of antenatal micronutrient supplementation in rural Nepal. J Nutr 141:1912–1917
icddr b, UNICEF Bangladesh,GAIN, Institute of public health and Nutrition (2013) National micronutrient status survey 2011–2012 final report. http://www.icddrb.org/publications/cat_view/10043-icddrb-documents/10058-icddrb-reports-and-working-papers/14275-survey-reports
Shamim A, Kabir A, Merrill R, Ali H, Rashid M, Schulze K, Labrique A, West K, Christian P (2013) Plasma zinc, vitamin B12 and α-tocopherol are positively and plasma γ-tocopherol is negatively associated with Hb concentration in early pregnancy in north-west Bangladesh. Public Health Nutr 16:1354–1361. doi:10.1017/S1368980013000475
Lindstrom E, Hossain MB, Lonnerdal B, Raqib R, El Arifeen S, Ekstrom EC (2011) Prevalence of anemia and micronutrient deficiencies in early pregnancy in rural Bangladesh, the MINIMat trial. Acta Obstet Gynecol Scand 90:47–56. doi:10.1111/j.1600-0412.2010.01014.x
Eneroth H, El Arifeen S, Persson L, Lönnerdal B, Hossain M, Stephensen C, Ekström E (2010) Maternal multiple micronutrient supplementation has limited impact on micronutrient status of Bangladeshi infants compared with standard iron and folic acid supplementation. J Nutr 140:618–624
Merrill RD, Shamim AA, Ali H, Jahan N, Labrique AB, Schulze K, Christian P, West KP (2011) Iron status of women is associated with the iron concentration of potable groundwater in rural Bangladesh. J Nutr 141:944–949
Tang AM, Graham NM, Chandra RK, Saah AJ (1997) Low serum vitamin B-12 concentrations are associated with faster human immunodeficiency virus type 1 (HIV-1) disease progression. J Nutr 127:345–351
Bunout D, Barrera G, Hirsch S, Gattas V, de la Maza MP, Haschke F, Steenhout P, Klassen P, Hager C, Avendano M (2004) Effects of a nutritional supplement on the immune response and cytokine production in free-living Chilean elderly. J Parenter Enteral Nutr (JPEN) 28:348–354
Fata FT, Herzlich BC, Schiffman G, Ast AL (1996) Impaired antibody responses to pneumococcal polysaccharide in elderly patients with low serum vitamin B12 levels. Ann Intern Med 124:299–304
Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, Omer SB, Shahid NS, Breiman RF, Steinhoff MC (2008) Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 359:1555–1564
Shahid NS, Steinhoff MC, Roy E, Begum T, Thompson CM, Siber GR (2002) Placental and breast transfer of antibodies after maternal immunization with polysaccharide meningococcal vaccine: a randomized, controlled evaluation. Vaccine 20:2404–2409
Watanabe F (2007) Vitamin B12 sources and bioavailability. Expl Biol Med 232:1266–1274
Ampola MG, Mahoney MJ, Nakamura E, Tanaka K (1975) Prenatal therapy of a patient with vitamin-B12-responsive methylmalonic acidemia. N Engl J Med 293:313–317
Kuzminski AM, Del Giacco EJ, Allen RH, Stabler SP, Lindenbaum J (1998) Effective treatment of cobalamin deficiency with oral cobalamin. Blood 92:1191–1198
Pedersen TL, Keyes WR, Shahab-Ferdows S, Allen LH, Newman JW (2011) Methylmalonic acid quantification in low serum volumes by UPLC–MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 879:1502–1506. doi:10.1016/j.jchromb.2011.03.039
Gilfix BM, Blank DW, Rosenblatt DS (1997) Novel reductant for determination of total plasma homocysteine. Clin Chem 43:687–688
Hampel DS-FS, Domeka JM, Siddiqua T, Raqib R, Allen LH (2014) Competitive chemiluminescent enzyme immunoassay for vitamin B12 analysis in human milk. Food Chem 153:60–65. doi:10.1016/j.foodchem.2013.12.033
WHO (1968) Nutritional anemia. World Health Organ Tech Rep Ser 405:5–37. World Health Organization, Geneva, Switzerland
Domellöf M, Dewey KG, Lönnerdal B, Cohen RJ, Hernell O (2002) The diagnostic criteria for iron deficiency in infants should be reevaluated. J Nutr 132:3680–3686
Allen RH, Stabler SP, Savage DG, Lindenbaum J (1990) Diagnosis of cobalamin deficiency I: usefulness of serum methylmalonic acid and total homocysteine concentrations. Am J Hematol 34:90–98
Cabot RC, Scully RE, Mark EJ, McNeely WF, McNeely BU, Jordan CD, Flood JG, Laposata M, Lewandrowski KB (1992) Normal reference laboratory values. New Engl J Med 327:718–724
Herrmann W, Schorr H, Obeid R, Geisel J (2003) Vitamin B-12 status, particularly holotranscobalamin II and methylmalonic acid concentrations, and hyperhomocysteinemia in vegetarians. Am J Clin Nutr 78:131–136
Schneede I, Dagnelie P, Van Staveren W, Vollset S, Refsum H, Ueland P (1994) Methylmalonic acid and homocysteine in plasma as indicators of functional cobalamin deficiency in infants on macrobiotic diets. Pediatr Res 36:194–201
Refsum H, Smith AD, Ueland PM, Nexo E, Clarke R, McPartlin J, Johnston C, Engbaek F, Schneede J, McPartlin C (2004) Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem 50:3–32
Vitamins and Mineral Nutrition Information System (2011) http://www.who.int/vmnis/indicators/serum_ferritin.pdf
Cook J, Skikne B, Baynes R (1993) Serum transferrin receptor. Annu Rev Med 44:63–74
Thurnham DI, Mburu AS, Mwaniki DL, Wagt AD (2005) Micronutrients in childhood and the influence of subclinical inflammation. Proc Nutr Soc 64:502–509
Calvin J, Neale G, Fotherby K, Price C (1988) The relative merits of acute phase proteins in the recognition of inflammatory conditions. Ann Clin Biochem 25:60–66
Baylin A, Villamor E, Rifai N, Msamanga G, Fawzi WW (2005) Effect of vitamin supplementation to HIV-infected pregnant women on the micronutrient status of their infants. Eur J Clin Nutr 59:960–968. doi:10.1038/sj.ejcn.1602201
Food and Nutrition Board IoM (1998) Dietary reference intakes: thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. National Academy Press, Washington
Herbert V (1987) Recommended dietary intakes (RDI) of vitamin B-12 in humans. Am J Clin Nutr 45:671–678
Duggan C, Srinivasan K, Thomas T et al (2014) Vitamin B-12 supplementation during pregnancy and early lactation increases maternal, breast milk, and infant measures of vitamin B-12 status. J Nutr 144:758–764. doi:10.3945/jn.113.187278
Yajnik C, Deshpande S, Jackson A, Refsum H, Rao S, Fisher D, Bhat D, Naik S, Coyaji K, Joglekar C (2008) Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia 51:29–38
Shahab-Ferdows SA-LM, Vergara-Castañeda H, Rosado JL, Keyes WR, Newman JW, Miller JW, Allen LH (2012) Vitamin B-12 supplementation of rural Mexican women changes biochemical vitamin B-12 status indicators but does not affect hematology or a bone turnover marker. J Nutr 142:1881–1887. doi:10.3945/jn.112.165712
Hay G, Clausen T, Whitelaw A, Trygg K, Johnston C, Henriksen T, Refsum H (2010) Maternal folate and cobalamin status predicts vitamin status in newborns and 6-month-old infants. J Nutr 140:557–564
Metz J (2008) A high prevalence of biochemical evidence of vitamin B12 or folate deficiency does not translate into a comparable prevalence of anemia. Food Nutr Bull 29:S74–S85
Henkle E, Steinhoff MC, Omer SB, Roy E, Arifeen SE, Raqib R, McNeal M, Breiman RF, Moss WJ, Zaman K (2011) Incidence of influenza virus infection in early infancy: a prospective study in South Asia. Pediatr Infect Dis J 30:170–173
Shahid N, Hoque S, Begum T, Steinhoff M, Thompson C, Siber G (1995) Serum, breast milk, and infant antibody after maternal immunisation with pneumococcal vaccine. The Lancet 346:1252–1257
Chu HY, Steinhoff MC, Magaret A, Zaman K, Roy E, Langdon G, Formica MA, Walsh EE, Englund JA (2014) Respiratory syncytial virus transplacental antibody transfer and kinetics in mother–infant pairs in Bangladesh. J Infect Dis 210:1582–1589. doi:10.1093/infdis/jiu316
Hitzig W, Dohmann U, Pluss H, Vischer D (1974) Hereditary transcobalamin II deficiency: clinical findings in a new family. J Pediatr 85:622–628
Hall CA, Green-Colligan PD, Begley JA (1981) The role of cobalamin in synthesis of protein and immunoglobulins by human peripheral lymphocytes. Nutr Res 1:349–361
Cv Dommelen, Slagboom G, Meester G, Wadman S (1963) Reversible hypogammaglobulinaemia in cyanocobalamin (B12) deficiency. Acta Med Scand 174:193–200
Kafetz K (1985) Immunoglobulin deficiency responding to vitamin B12 in two elderly patients with megaloblastic anaemia. Postgrad Med J 61:1065–1066
Erkurt MA, Aydogdu I, Dikilitaş M, Kuku I, Kaya E, Bayraktar N, Ozhan O, Ozkan I, Sönmez A (2008) Effects of cyanocobalamin on immunity in patients with pernicious anemia. Med Princ Pract 17:131–135
Greibe E, Lildballe DL, Streym S, Vestergaard P, Rejnmark L, Mosekilde L, Nexo E (2013) Cobalamin and haptocorrin in human milk and cobalamin-related variables in mother and child: a 9-mo longitudinal study. Am J Clin Nutr 98:389–395
West L (2002) Defining critical windows in the development of the human immune system. Hum Exp Toxicol 21:499–505
Prendergast AJ, Rukobo S, Chasekwa B, Mutasa K, Ntozini R, Mbuya MN, Jones A, Moulton LH, Stoltzfus RJ, Humphrey JH (2014) Stunting is characterized by chronic inflammation in Zimbabwean infants. PLoS One 9:e86928
Acknowledgments
We wish to thank Dr. Md. Sirajul Islam and Dr. Chinmoy K. Das of MCHTI for the generous support and creation of a congenial atmosphere to conduct the study. We are indebted to the women who participated in this project, to the field staff, laboratory technicians, hospital nurses and staffs who made the study possible. We gratefully appreciate the help of Md. Shahidul Islam with statistical analysis. Dietary diversity questionnaire was obtained from Dr. Kuntal Kumar Saha (formerly at ICDDR,B; currently at International Food Policy Research Institute). The B12 and placebo pills were kind donation of Incepta Pharmaceuticals Ltd. The study was funded by the Nestle Foundation, (GR-00745), Swedish International Development Cooperation Agency (GR-00599), Bill & Melinda Gates Foundation (OPP1061055) and intramural USDA-ARS Project (5306-51000-003-00D).
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
ClinicalTrials.gov identifier NCT01795131.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Siddiqua, T.J., Ahmad, S.M., Ahsan, K.B. et al. Vitamin B12 supplementation during pregnancy and postpartum improves B12 status of both mothers and infants but vaccine response in mothers only: a randomized clinical trial in Bangladesh. Eur J Nutr 55, 281–293 (2016). https://doi.org/10.1007/s00394-015-0845-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0845-x