Abstract
Background and purpose
Agar contains a high amount of soluble fibre and has been shown to delay gastric emptying (GE) without impacting on glycaemic response (GR). The current study aimed to further the limited data on the effect of agar on metabolism by assessing the effects on GE and GR as well as appetite- and diet-induced thermogenesis (DIT).
Methods
In this randomized control trial, eleven healthy volunteers were tested on two occasions following an overnight fast. Following baseline and resting measurements, volunteers were either fed a fruit-flavoured drink (liquid) or consumed a fruit-flavoured jelly (jelly). The two were exactly the same in composition except the jelly contained 4 g of agar crystals. Both contained 50 g of available carbohydrate. DIT was measured using indirect calorimetry, GE using the 13C sodium acetate breath test, appetite using visual analogue scale and GR using finger prick blood samples.
Results
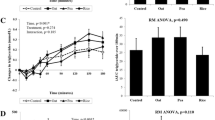
The jelly significantly delayed GE across all time points—latency phase (p = 0.07), lag phase (p = 0.04), half-time (p < 0.0001), ascension time (p = 0.025). The jelly also increased all appetite parameters—hunger (p = 0.006), fullness (p = 0.035), desire to eat (p = 0.03) and prospective consumption (p = 0.011). However, there were no significant differences in either GR or postprandial DIT between the liquid and jelly.
Conclusion
Agar delays GE and increases appetite but does not change GR or DIT most probably due to the increase in viscosity caused by the agar jelly.



Similar content being viewed by others
References
Horowitz M, Edelbroek MA, Wishart JM, Straathof JW (1993) Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia 36:857–862
Clegg ME, Thondre PS (2012) The influence of barley β-glucan on satiety, glycaemic response and energy expenditure. Proc Nutr Soc 71:E72
Maeda H, Yamamoto R, Hirao K, Tochikubo O (2005) Effects of agar (kanten) diet on obese patients with impaired glucose tolerance and type 2 diabetes. Diabetes Obes Metab 7:40–46
Sanaka M, Yamamoto T, Anjiki H, Nagasawa K, Kuyama Y (2007) Effects of agar and pectin on gastric emptying and post-prandial glycaemic profiles in healthy human volunteers. Clin Exp Pharmacol Physiol 34:1151–1155
Clegg ME, Ranawana V, Shafat A, Henry CJ (2013) Soups increase satiety through delayed gastric emptying yet increased glycaemic response. Eur J Clin Nutr 67:8–11
Ahmad A, Anjum FM, Zahoor T, Nawaz H, Dilshad SM (2012) Beta glucan: a valuable functional ingredient in foods. Crit Rev Food Sci Nutr 52:201–212
Jenkins DJA, Marchie A, Augustin LSA, Ros E, Kendall CWC (2004) Viscous dietary fibre and metabolic effects. Clin Nutr Suppl 1:39–49
Bornet FR, Jardy-Gennetier AE, Jacquet N, Stowell J (2007) Glycaemic response to foods: impact on satiety and long-term weight regulation. Appetite 49:535–553
Mayer J (1955) Regulation of energy intake and the body weight: the glucostatic theory and the lipostatic hypothesis. Ann N Y Acad Sci 63:15–43
Cecil JE, Francis J, Read NW (1998) Relative contributions of intestinal, gastric, oro-sensory influences and information to changes in appetite induced by the same liquid meal. Appetite 31:377–390
Geliebter A (1988) Gastric distension and gastric capacity in relation to food intake in humans. Physiol Behav 44:665–668
Tarini J, Wolever TM (2010) The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl Physiol Nutr Metab 35:9–16
Johansson EV, Nilsson AC, Ostman EM, Bjorck IM (2013) Effects of indigestible carbohydrates in barley on glucose metabolism, appetite and voluntary food intake over 16 h in healthy adults. Nutr J 12:46
Westerterp KR (2004) Diet induced thermogenesis. Nutr Metab 1:5
Heijnen ML, Deurenberg P, van Amelsvoort JM, Beynen AC (1995) Replacement of digestible by resistant starch lowers diet-induced thermogenesis in healthy men. Br J Nutr 73:423–432
Raben A, Christensen NJ, Madsen J, Holst JJ, Astrup A (1994) Decreased postprandial thermogenesis and fat oxidation but increased fullness after a high-fiber meal compared with a low-fiber meal. Am J Clin Nutr 59:1386–1394
Reed GW, Hill JO (1996) Measuring the thermic effect of food. Am J Clin Nutr 63:164–169
Lusk G (1928) The elements of the science of nutrition. WB Saunders Co, London
Stubbs RJ, Hughes DA, Johnstone AM, Rowley E, Reid C, Elia M, Stratton R, Delargy H, King N, Blundell JE (2000) The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr 84:405–415
Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G, Wolever TM (2005) Glycaemic index methodology. Nutr Res Rev 18:145–171
FAO/WHO (1998) Carbohydrates in human nutrition. Report of a joint FAO/WHO expert consultation. FAO Food Nutr Pap 66:1–140
Stork AD, Kemperman H, Erkelens DW, Veneman TF (2005) Comparison of the accuracy of the HemoCue glucose analyzer with the Yellow Springs Instrument glucose oxidase analyzer, particularly in hypoglycemia. Eur J Endocrinol 153:275–281
Wolever TMS (2006) The glycaemic index : a physiological classification of dietary carbohydrate. CABI, Oxfordshire
Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX (1995) The use of areas under curves in diabetes research. Diabetes Care 18:245–250
Braden B, Adams S, Duan LP, Orth KH, Maul FD, Lembcke B, Hor G, Caspary WF (1995) The [13C]acetate breath test accurately reflects gastric emptying of liquids in both liquid and semisolid test meals. Gastroenterology 108:1048–1055
Haycock GB, Schwartz GJ, Wisotsky DH (1978) Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr 93:62–66
Ghoos YF, Maes BD, Geypens BJ, Mys G, Hiele MI, Rutgeerts PJ, Vantrappen G (1993) Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology 104:1640–1647
Jackson SJ, Leahy FE, McGowan AA, Bluck LJ, Coward WA, Jebb SA (2004) Delayed gastric emptying in the obese: an assessment using the non-invasive (13)C-octanoic acid breath test. Diabetes Obes Metab 6:264–270
Schommartz B, Ziegler D, Schadewaldt P (1998) Significance of diagnostic parameters in [13C]octanoic acid gastric emptying breath tests. Isotopes Environ Health Stud 34:135–143
Thondre PS, Henry CJ (2009) High-molecular-weight barley beta-glucan in chapatis (unleavened Indian flatbread) lowers glycemic index. Nutr Res 29:480–486
Blackburn NA, Redfern JS, Jarjis H, Holgate AM, Hanning I, Scarpello JH, Johnson IT, Read NW (1984) The mechanism of action of guar gum in improving glucose tolerance in man. Clin Sci 66:329–336
de Wijk RA, Zijlstra N, Mars M, de Graaf C, Prinz JF (2008) The effects of food viscosity on bite size, bite effort and food intake. Physiol Behav 95:527–532
Kristensen M, Jensen MG (2011) Dietary fibres in the regulation of appetite and food intake. Importance of viscosity. Appetite 56:65–70
Kissileff HR, Carretta JC, Geliebter A, Pi-Sunyer FX (2003) Cholecystokinin and stomach distension combine to reduce food intake in humans. Am J Physiol Regul Integr Comp Physiol 285:R992–R998
Cassady BA, Considine RV, Mattes RD (2012) Beverage consumption, appetite, and energy intake: what did you expect? Am J Clin Nutr 95:587–593
Conflicts of interest
There are no conflicts of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clegg, M.E., Shafat, A. The effect of agar jelly on energy expenditure, appetite, gastric emptying and glycaemic response. Eur J Nutr 53, 533–539 (2014). https://doi.org/10.1007/s00394-013-0559-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-013-0559-x