Abstract
Purpose
High level of microsatellite instability (MSI-H) in colorectal cancer (CRC) is caused by the inactivation of mismatch repair (MMR) genes; however, it is unknown for tumors with low level MSI (MSI-L). The protein complex involving MSH3 preferentially recognizes insertion/deletion loops (IDLs) of two to eight bases and di- and tetranucleotide repeats are affected in the majority of MSI-L CRC.
Methods
We selected 10 and eight MSI-L CRCs from 228 and 204 patients with sporadic and hereditary disease, respectively. The tumors were analyzed for protein expression of MSH3, MSH2, MSH6, MLH1, and PMS2, and for mutations and loss of heterozygosity (LOH) in MSH3.
Results
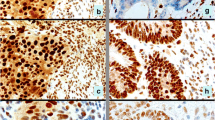
Four tumors showed a markedly reduced MSH3 expression, whereas all 18 tumors had normal expression of the remaining MMR proteins. Twenty-five different sequence variants were identified. None of these results in a truncated protein, though L902W represents the first constitutional missense mutation in MSH3 predicted to be functional based on conservation among mutS homologues. All variants have also been found in normal DNA of the patients and in controls. LOH intragenic to MSH3 was evident for 12 of 16 (75%) informative tumors.
Conclusions
Occurrence of sequence variants in normal DNA of the patients and in controls excludes somatic mutations and mutations specific to the CRC patient population, respectively. In contrast, the high frequency of LOH as well as the aberrant protein expression in some tumors indicates an involvement of MSH3 impairment in MSI-L CRC.



Similar content being viewed by others
References
Jiricny J (2006) The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol 7:335–346
Jascur T, Boland CR (2006) Structure and function of the components of the human DNA mismatch repair system. Int J Cancer 119:2030–2035
Chang DK, Ricciardiello L, Goel A, Chang CL, Boland CR (2000) Steady-state regulation of the human DNA mismatch repair system. J Biol Chem 275:18424–18431
Bhattacharyya NP, Skandalis A, Ganesh A, Groden J, Meuth M (1994) Mutator phenotypes in human colorectal carcinoma cell lines. Proc Natl Acad Sci U S A 91:6319–6323
Papadopoulos N, Nicolaides NC, Liu B, Parsons R, Lengauer C, Palombo F, D’Arrigo A, Markowitz S, Willson JKV, Kinzler KW, Jiricny J, Vogelstein B (1995) Mutations of GTBP in genetically unstable cells. Science 268:1915–1917
Zhang Y, Yuan F, Presnell SR, Tian K, Gao Y, Tomkinson AE, Gu L, Li GM (2005) Reconstitution of 5′-directed human mismatch repair in a purified system. Cell 122:693–705
Acharya S, Wilson T, Gradia S, Kane MF, Guerrette S, Marsischky GT, Kolodner R, Fishel R (1996) hMSH2 forms specific mispair-binding complexes with hMSH3 and hMSH6. Proc Natl Acad Sci U S A 93:13629–13634
Genschel J, Littman SJ, Drummond JT, Modrich P (1998) Isolation of MutSbeta from human cells and comparison of the mismatch repair specificities of MutSbeta and MutSalpha. J Biol Chem 273:19895–19901
Umar A, Risinger JI, Glaab WE, Tindall KR, Barrett JC, Kunkel TA (1998) Functional overlap in mismatch repair by human MSH3 and MSH6. Genetics 148:1637–1646
Aaltonen LA, Peltomaki P, Leach FS, Sistonen P, Pylkkanen L, Mecklin JP, Jarvinen H, Powell SM, Jen J, Hamilton SR (1993) Clues to the pathogenesis of familial colorectal cancer. Science 260:812–816
Thibodeau SN, Bren G, Schaid D (1993) Microsatellite instability in cancer of the proximal colon. Science 260:816–819
Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M (1993) Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 363:558–561
Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S (1998) A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 58:5248–5257
Kane MF, Loda M, Gaida GM, Lipman J, Mishra R, Goldman H, Jessup JM, Kolodner R (1997) Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res 57:808–811
de la Chapelle A (2004) Genetic predisposition to colorectal cancer. Nat Rev Cancer 4:769–780
Lynch HT, de la Chapelle A (2003) Hereditary colorectal cancer. N Engl J Med 348:919–932
Rodriguez-Bigas MA, Boland CR, Hamilton SR, Henson DE, Jass JR, Khan PM, Lynch H, Perucho M, Smyrk T, Sobin L, Srivastava S (1997) A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst 89:1758–1762
Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, Fishel R, Lindor NM, Burgart LJ, Hamelin R, Hamilton SR, Hiatt RA et al (2004) Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 96:261–268
Plaschke J, Krüger S, Pistorius S, Theissig F, Saeger HD, Schackert HK (2002) Involvement of hMSH6 in the development of hereditary and sporadic colorectal cancer revealed by immunostaining is based on germline mutations, but rarely on somatic inactivation. Int J Cancer 97:643–648
Jiricny J, Marra G (2003) DNA repair defects in colon cancer. Curr Opin Genet Dev 13:61–69
Peltomaki P (2005) Lynch syndrome genes. Familial Cancer 4:227–232
Dietmaier W, Wallinger S, Bocker T, Kullmann F, Fishel R, Ruschoff J (1997) Diagnostic microsatellite instability: definition and correlation with mismatch repair protein expression. Cancer Res 57:4749–4756
Plaschke J, Kruppa C, Tischler R, Bocker T, Pistorius S, Dralle H, Ruschoff J, Saeger HD, Fishel R, Schackert HK (2000) Sequence analysis of the mismatch repair gene hMSH6 in the germline of patients with familial and sporadic colorectal cancer. Int J Cancer 85:606–613
Wu Y, Berends MJ, Mensink RG, Kempinga C, Sijmons RH, Der Zee AG, Hollema H, Kleibeuker JH, Buys CH, Hofstra RM (1999) Association of hereditary nonpolyposis colorectal cancer-related tumors displaying low microsatellite instability with MSH6 germline mutations. Am J Hum Genet 65:1291–1298
Plaschke J, Kruger S, Jeske B, Theissig F, Kreuz FR, Pistorius S, Saeger HD, Iaccarino I, Marra G, Schackert HK (2004) Loss of MSH3 protein expression is frequent in MLH1-deficient colorectal cancer and is associated with disease progression. Cancer Res 64:864–870
Kleczkowska HE, Marra G, Lettieri T, Jiricny J (2001) hMSH3 and hMSH6 interact with PCNA and colocalize with it to replication foci. Genes Dev 15:724–736
Nakajima E, Orimo H, Ikejima M, Shimada T (1995) Nine-bp repeat polymorphism in exon 1 of the hMSH3 gene. Jpn J Hum Genet 40:343–345
Cawkwell L, Lewis FA, Quirke P (1994) Frequency of allele loss of DCC, p53, RBI, WT1, NF1, NM23 and APC/MCC in colorectal cancer assayed by fluorescent multiplex polymerase chain reaction. Br J Cancer 70:813–818
Ng PC, Henikoff S (2001) Predicting deleterious amino acid substitutions. Genome Res 11:863–874
Jass JR, Biden KG, Cummings MC, Simms LA, Walsh M, Schoch E, Meltzer SJ, Wright C, Searle J, Young J, Leggett BA (1999) Characterisation of a subtype of colorectal cancer combining features of the suppressor and mild mutator pathways. J Clin Pathol 52:455–460
Jass JR, Young J, Leggett BA (2001) Biological significance of microsatellite instability-low (MSI-L) status in colorectal tumors. Am J Pathol 158:779–781
Halford SE, Sawyer EJ, Lambros MB, Gorman P, Macdonald ND, Talbot IC, Foulkes WD, Gillett CE, Barnes DM, Akslen LA, Lee K, Jacobs IJ et al (2003) MSI-low, a real phenomenon which varies in frequency among cancer types. J Pathol 201:389–394
Laiho P, Launonen V, Lahermo P, Esteller M, Guo M, Herman JG, Mecklin JP, Jarvinen H, Sistonen P, Kim KM, Shibata D, Houlston RS et al (2002) Low-level microsatellite instability in most colorectal carcinomas. Cancer Res 62:1166–1170
Brinkmann B, Klintschar M, Neuhuber F, Huhne J, Rolf B (1998) Mutation rate in human microsatellites: influence of the structure and length of the tandem repeat. Am J Hum Genet 62:1408–1415
Tomlinson I, Halford S, Aaltonen L, Hawkins N, Ward R (2002) Does MSI-low exist? J Pathol 197:6–13
Halford S, Sasieni P, Rowan A, Wasan H, Bodmer W, Talbot I, Hawkins N, Ward R, Tomlinson I (2002) Low-level microsatellite instability occurs in most colorectal cancers and is a nonrandomly distributed quantitative trait. Cancer Res 62:53–57
Kambara T, Matsubara N, Nakagawa H, Notohara K, Nagasaka T, Yoshino T, Isozaki H, Sharp GB, Shimizu K, Jass J, Tanaka N (2001) High frequency of low-level microsatellite instability in early colorectal cancer. Cancer Res 61:7743–7746
Haugen AC, Goel A, Yamada K, Marra G, Nguyen TP, Nagasaka T, Kanazawa S, Koike J, Kikuchi Y, Zhong X, Arita M, Shibuya K, Oshimura M, Hemmi H, Boland CR, Koi M (2008) Genetic instability caused by loss of MutS homologue 3 in human colorectal cancer. Cancer Res 68:8465–8472
Orimo H, Nakajima E, Yamamoto M, Ikejima M, Emi M, Shimada T (2000) Association between single nucleotide polymorphisms in the hMSH3 gene and sporadic colon cancer with microsatellite instability. J Hum Genet 45:228–230
Berndt SI, Platz EA, Fallin MD, Thuita LW, Hoffman SC, Helzlsouer KJ (2007) Mismatch repair polymorphisms and the risk of colorectal cancer. Int J Cancer 120:1548–1554
Obmolova G, Ban C, Hsieh P, Yang W (2000) Crystal structures of mismatch repair protein MutS and its complex with a substrate DNA. Nature 12(407):703–710
Kawakami T, Shiina H, Igawa M, Deguchi M, Nakajima K, Ogishima T, Tokizane T, Urakami S, Enokida H, Miura K, Ishii N, Kane CJ et al (2004) Inactivation of the hMSH3 mismatch repair gene in bladder cancer. Biochem Biophys Res Commun 325:934–942
Kim HG, Lee S, Kim DY, Ryu SY, Joo JK, Kim JC, Lee KH, Lee JH (2010) Aberrant methylation of DNA mismatch repair genes in elderly patients with sporadic gastric carcinoma: a comparison with younger patients. J Surg Oncol 101:28–35
Huang J, Kuismanen SA, Liu T, Chadwick RB, Johnson CK, Stevens MW, Richards SK, Meek JE, Gao X, Wright FA, Mecklin JP, Jarvinen HJ, Gronberg H, Bisgaard ML, Lindblom A, Peltomaki P (2001) MSH6 and MSH3 are rarely involved in genetic predisposition to nonpolypotic colon cancer. Cancer Res 61:1619–1623
Benachenhou N, Guiral S, Gorska-Flipot I, Labuda D, Sinnett D (1998) High resolution deletion mapping reveals frequent allelic losses at the DNA mismatch repair loci hMLH1 and hMSH3 in non-small cell lung cancer. Int J Cancer 77:173–180
Benachenhou N, Guiral S, Gorska-Flipot I, Labuda D, Sinnett D (1999) Frequent loss of heterozygosity at the DNA mismatch-repair loci hMLH1 and hMSH3 in sporadic breast cancer. Br J Cancer 79:1012–1017
Berends MJ, Wu Y, Sijmons RH, Mensink RG, van der Sluis T, Hordijk-Hos JM, de Vries EG, Hollema H, Karrenbeld A, Buys CH, van der Zee AG, Hofstra RM, Kleibeuker JH (2002) Molecular and clinical characteristics of MSH6 variants: an analysis of 25 index carriers of a germline variant. Am J Hum Genet 70:26–37
Sugawara N, Paques F, Colaiacovo M, Haber JE (1997) Role of Saccharomyces cerevisiae Msh2 and Msh3 repair proteins in double-strand break-induced recombination. Proc Natl Acad Sci U S A 94:9214–9219
Claij N, te Riele H (1999) Microsatellite instability in human cancer: a prognostic marker for chemotherapy? Exp Cell Res 246:1–10
Carethers JM, Smith EJ, Behling CA, Nguyen L, Tajima A, Doctolero RT, Cabrera BL, Goel A, Arnold CA, Miyai K, Boland CR (2004) Use of 5-fluorouracil and survival in patients with microsatellite-unstable colorectal cancer. Gastroenterology 126:394–401
Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, Hamilton SR, Laurent-Puig P, Gryfe R, Shepherd LE, Tu D, Redston M, Gallinger S (2003) Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 349:247–257
Acknowledgments
We thank Ms. Reichmann and Ms. Colditz for excellent technical assistance. We would like to thank Prof. J. Jiricny and Dr. G. Marra (Institute for Molecular Cancer Research, University of Zurich, Switzerland) for kindly providing the polyclonal MSH3 antibody. This work was supported in part by the Wilhelm Sander Stiftung Grant 2005.062.1 and the Deutsche Forschungsgemeinschaft Grant PL 311/1-1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plaschke, J., Preußler, M., Ziegler, A. et al. Aberrant protein expression and frequent allelic loss of MSH3 in colorectal cancer with low-level microsatellite instability. Int J Colorectal Dis 27, 911–919 (2012). https://doi.org/10.1007/s00384-011-1408-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-011-1408-0