Abstract
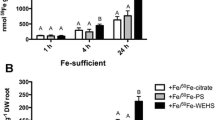
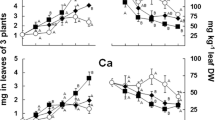
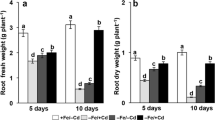
The aim of this work is to evaluate the capability of tomato plants to use different Fe sources, such as Fe citrate, Fe phytosiderophores, and Fe complexed by a water-extractable humic substances (Fe-WEHS) also in relation to physiological and molecular adaptations induced by these complexes at the root level. Tomato plants acquired higher amounts of Fe from Fe-WEHS than from the other two sources and this phenomenon occurred only when the treatment lasted 24 h. The higher acquisition of Fe from Fe-WEHS than other sources depended on a reductive mechanism and on rhizosphere acidification and appeared to be due neither to a higher apoplastic loading nor to a higher resistance of WEHS to microbial degradation. Supply of the different Fe complexes to deficient plants induced a transient upregulation of Fe(III)-chelate reductase (LeFRO1) and Fe transporter genes, LeIRT1 and LeIRT2. In Fe-WEHS-fed plants, where a quicker and higher upregulation of these genes was evident, a coordination in the expression of LeFRO1, LeIRT1, and LeIRT2 genes occurred already after 1 h treatment when the amount of Fe acquired by the plants from the three sources was similar. Iron from Fe-WEHS could be efficiently acquired in a mixture of natural Fe complexes possibly occurring in the rhizosphere. This phenomenon is due to an altered expression of Fe uptake-related genes and to the root capacity to create favorable conditions for the micronutrient uptake into the rhizosphere.










Similar content being viewed by others
References
Aguirre E, Lemenager D, Bacaicoa E, Fuentes M, Baigorri M, Zamarreno AM, Garcia-Mina JM (2009) The root application of a purified leonardite humic acid modifies the transcriptional regulation of the main physiological root responses to Fe deficiency in Fe-sufficient cucumber plants. Plant Physiol Bioch 47:215–223
Aiken GR, Thurman EM, Malcolm R (1979) Comparison of XAD macroporous resin for the concentration of fulvic acid from aqueous solution. Anal Chem 51:1799–1803
Amman C, Amberger A (1989) Phosphorus efficiency of buckwheat (Fagopyron esculentum). J Plant Nutr Soil Sc 52:181–189
Barak P, Chen YN (1992) Equivalent radii of humic macromolecules from acid–base titration. Soil Sci 154:184–195
Bereczky Z, Wang HY, Schubert V, Ganal M, Bauer P (2003) Differential regulation of nramp and irt metal transporter genes in wild type and iron uptake mutants of tomato. J Biol Chem 278:24697–24704
Bienfait HF, van den Briel W, Mesland-Mul NT (1985) Free space iron pools in roots: generation and mobilization. Plant Physiol 78:596–600
Brown JC, Ambler JE (1974) Iron-stress response in tomato (Lycopersicon esculentum) 1. Sites of Fe reduction, absorption and transport. Physiol Plant 31:221–224
Brown JC, Chaney RL, Ambler JE (1971) A new tomato mutant inefficient in the transport of iron. Physiol Plant 25:48–53
Buckhout TJ, Yang TJ, Schmidt W (2009) Early iron-deficiency-induced transcriptional changes in Arabidopsis roots as revealed by microarray analyses. BMC Genom 10:147
Cesco S, Römheld V, Varanini Z, Pinton R (2000) Solubilization of iron by water-extractable humic substances. J Plant Nutr Soil Sci 163:285–290
Cesco S, Nikolic M, Römheld V, Varanini Z, Pinton R (2002) Uptake of 59Fe from soluble 59Fe-humate complexes by cucumber and barley plants. Plant Soil 241:121–128
Cesco S, Rombola AD, Tagliavini M, Varanini Z, Pinton R (2006) Phytosiderophores released by graminaceous species promote Fe59-uptake in citrus. Plant Soil 287:223–233
Cesco S, Neumann G, Tomasi N, Pinton R, Weisskopf L (2010) Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 329:1–25
Cesco S, Mimmo T, Tonon G, Tomasi N, Pinton R, Terzano R, Neumann G, Weisskopf L (2012) Plant-borne flavonoids released into the rhizosphere: impact on soil bio-activities related to plant nutrition. A review. Biol Fertil Soils 48:123–149
Chen Y (1996) Organic matter reactions involving micro-nutrients in soils and their effect on plants. In: Piccolo A (ed) Humic substances in terrestrial ecosystems. Elsevier Science B.V, Amsterdam, pp 507–529
Chen Y, Clapp CE, Magen H (2004) Mechanism of plant growth stimulation by humic substances: the role of organo–iron complexes. Soil Sci Plant Nutr 50:1089–1095
Cohen CK, Norvell WA, Kochian LV (1997) Induction of the root cell plasma membrane ferric reductase: an exclusive role for Fe and Cu. Plant Physiol 114:1061–1069
Colombo C, Palumbo G, Sellitto VM, Rizzardo C, Tomasi N, Pinton R, Cesco S (2012) Characteristics of insoluble, high molecular weight Fe-humic substances used as plant Fe sources. Soil Sci Soc Am J. doi:10.2136/sssaj11.0393
Connolly EL, Fett JP, Guerinot ML (2002) Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell 14:1347–1357
Connolly EL, Campbell NH, Grotz N, Prichard CL, Guerinot ML (2003) Overexpression of the FRO2 ferric chelate reductase confers tolerance to growth on low iron and uncovers posttranscriptional control. Plant Physiol 133:1102–1110
Crowley D, Kraemer MS (2007) Function of siderophores in the plant rhizosphere. In: Pinton R, Varanini Z, Nannipieri P (eds) The rhizosphere: biochemistry and organic substances at the soil–plant interface, 2nd edn. CRC, Boca Raton, pp 173–200
Eckhardt U, Mas MA, Buckhout TJ (2001) Two iron-regulated cation transporters from tomato complement metal uptake-deficient yeast mutants. Plant Mol Biol 45:437–448
Gerke J (1993) Solubilization of Fe(III) from humic-Fe complexes, humic/Fe-oxide mixtures and from poorly ordered Fe-oxide by organic-acids consequences for P-adsorption. J Plant Nutr Soil Sc 156:253–257
Guzman G, Alcantara E, Barron V, Torrent J (1994) Phytoavailability of phosphate adsorbed on ferrihydrite, hematite, and goethite. Plant Soil 159:219–225
Hördt W, Römheld V, Winkelmann G (2000) Fusarinines and dimerum acid, mono- and dihydroxamate siderophores from Penicillum chrysogenum improve iron utilization by strategy I and strategy II plants. Biometals 13:37–46
Howe JA, Choi YH, Loeppert RH, Wei LC, Senseman SA, Juo ASR (1999) Column chromatography and verification of phytosiderophores by phenylisothiocyanate derivatization and UV detection. J Chromatogr A 841:155–164
Jin CW, You GY, He YF, Tang CX, Wu P, Zheng SJ (2007) Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. Plant Physiol 144:278–285
Jones DL (1998) Organic acids in the rhizosphere—a critical review. Plant Soil 205:25–44
Jones DL, Darrah P (1994) Role of root derived organic acid in the mobilization of nutrients from the rhizosphere. Plant Soil 166:247–257
Jones DL, Darrah P, Kochian L (1996) Critical evaluation of organic acid mediated iron dissolution in the rhizosphere and its potential role in root iron uptake. Plant Soil 180:57–66
Lindsay WL, Schwab AP (1982) The chemistry of iron in soils and its availability to plants. J Plant Nutr 5:821–840
Ma JF, Nomoto K (1996) Effective regulation of iron acquisition in graminaceous plants. The role of mugineic acids as phytosiderophores. Physiol Plant 97:609–617
Ma JF, Taketa S, Chang YC, Iwashita T, Matsumoto H, Takeda K, Nomoto K (1999) Genes controlling hydroxylations of phytosiderophores are located on different chromosomes in barley (Hordeum vulgare L.). Planta 207:590–596
Maniatis T, Sambrook J, Fritsch EF (1982) Molecular Cloning: A laboratory manual. Cold Spring Harbor Laboratory, New York
Marschner H (1995) Mineral nutrition of higher plants. Academic, London
Neilands JB (1981) Iron absorption and transport in microorganisms. Annu Rev Nutr 1:27–46
Neumann G, Röhmeld V (2007) The release of root exudates as affected by the plant physiological status. In: Pinton R, Varanini Z, Nannipieri P (eds) The rhizosphere: biochemistry and organic substances at the soil–plant interface, 2nd edn. CRC, Boca Raton, pp 23–72
Piccolo A, Stevenson FJ (1981) Infrared spectra of Cu2+, Pb2+ and Ca2+ complexes of soil humic substances. Geoderma 27:195–208
Pinton R, Cesco S, De Nobili M, Santi S, Varanini Z (1997a) Water- and pyrophosphate-extractable humic substances fractions as a source of iron for Fe-deficient cucumber plants. Biol Fert Soils 26:23–27
Pinton R, Cesco S, Santi S, Varanini Z (1997b) Soil humic substances stimulate proton release by intact oat seedling roots. J Plant Nutr 20:857–869
Pinton R, Cesco S, Santi S, Agnolon F, Varanini Z (1999a) Water-extractable humic substances enhance iron deficiency responses by Fe-deficient cucumber plants. Plant Soil 210:145–157
Pinton R, Cesco S, Iacolettig G, Astolfi S, Varanini Z (1999b) Modulation of NO -3 uptake by water-extractable humic substances: involvement of root plasma membrane H(+)ATPase. Plant Soil 215:155–161
Reichard PU, Kraemer SM, Frazier SW, Kretzschmar R (2005) Goethite dissolution in the presence of phytosiderophores: rates, mechanisms, and the synergistic effect of oxalate. Plant Soil 276:115–132
Ritz C, Spiess AN (2008) qpcR: an R package for sigmoidal model selection in quantitative real-time polymerase chain reaction analysis. Bioinformatics 24:1549–1551
Römheld V (1987) Existence of two different strategies for the acquisition of iron in higher plants. In: Winkelmann G, van der Helm D, Neilands JB (eds) Iron transport in animal, plants and microorganisms. VCH Chemie, Weinheim, pp 353–374
Römheld V, Marschner H (1986a) Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol 80:175–180
Römheld V, Marschner H (1986b) Mobilization of iron in the rhizosphere of different plant species. In: Tinker B, Laüchli A (eds) Advances in plant nutrition. Praeger Scientific, New York, pp 155–204
Schmidt W, Janiesch P (1991) Ferric reduction by Geum urbanum—a kinetic study. J Plant Nutr 14:1023–1034
Stevenson FJ (1994) Humus chemistry: genesis, composition, reactions. Wiley, New York, pp 1–496
Sugiura Y, Tanaka H, Mino Y, Ishida T, Ota N, Inoue M, Nomoto K, Yoshioka H, Takemoto T (1981) Structure, properties, and transport mechanism of iron(III) complex of mugineic acid, a possible phytosiderophore. J Chem Soc 103:6979–6982
Takagi S, Nomoto K, Takemoto T (1984) Physiological aspect of mugineic acid, a possible phytosiderophore of graminaceous plants. J Plant Nutr 7:469–477
Tomasi N, Weisskopf L, Renella G, Landi L, Pinton R, Varanini Z, Nannipieri P, Torrent J, Martinoia E, Cesco S (2008) Flavonoids of white lupin roots participate in phosphorus mobilization from soil. Soil Biol Biochem 40:1971–1974
Tomasi N, Kretzschmar T, Espen L, Weisskopf L, Fuglsang AT, Palmgren MG, Neumann G, Varanini Z, Pinton R, Martinoia E, Cesco S (2009a) Plasma membrane H+-ATPase-dependent citrate exudation from cluster roots of phosphate-deficient white lupin. Plant Cell Environ 32:465–475
Tomasi N, Monte R, Rizzardo C, Venuti S, Zamboni A, Cesco S, Pinton R, Varanini Z (2009b) Effects of water-extractable humic substances on molecular physiology of nitrate uptake in two maize inbred lines with different nitrogen use efficiency. The Proceedings of the International Plant Nutrition Colloquium XVI 1243
Tomasi N, Rizzardo C, Monte R, Gottardi S, Jelali N, Terzano R, Vekemans B, De Nobili M, Varanini Z, Pinton R, Cesco S (2009c) Micro-analytical, physiological and molecular aspects of Fe acquisition in leaves of Fe-deficient tomato plants re-supplied with natural Fe-complexes in nutrient solution. Plant Soil 325:25–38
van Hees PAW, Lundstrom US (2000) Equilibrium models of aluminium and iron complexation with different organic acids in soil solution. Geoderma 94:201–221
Vansuyt G, Robin A, Briat JF, Curie C, Lemanceau P (2007) Iron acquisition from Fe-pyoverdine by Arabidopsis thaliana. Mol Plant Microbe In 20:441–447
Varanini Z, Pinton R, De Biasi MG, Astolfi S, Maggioni A (1993) Low molecular weight humic substances stimulate H+-ATPase activity of plasma membrane vesicles isolated from oat (Avena sativa L.) roots. Plant Soil 153:61–69
Vert G, Barberon M, Zelazny E, Séguéla M, Briat JF, Curie C (2009) Arabidopsis IRT2 cooperates with the high-affinity iron uptake system to maintain iron homeostasis in root epidermal cells. Planta 229:1171–1179
vonWirén N, Mori S, Marschner H, Römheld V (1994) Iron inefficiency in maize mutant ys1 (Zea mays L cv yellow-stripe) is caused by a defect in uptake of iron phytosiderophores. Plant Physiol 106:71–77
Walter A, Pich A, Scholz G, Marschner H, Romheld V (1995) Effects of iron nutritional-status and time of day on concentrations of phytosiderophores and nicotinamine in different root and shoot zones of barley. J Plant Nutr 18:1577–1593
Wang Y, Brown HN, Crowley DE, Szaniszlo PJ (1993) Evidence for direct utilization of a siderophore, ferrioxamine B, in axenically grown cucumber. Plant Cell Environ 16:579–585
Weisskopf L, Abou-Mansour E, Fromin N, Tomasi N, Santelia D, Edelkott I, Neumann G, Aragno M, Tabacchi R, Martinoia E (2006) White lupin has developed a complex strategy to limit microbial degradation of secreted citrate required for phosphate acquisition. Plant Cell Environ 29:919–927
Yehuda Z, Shenker M, Römheld V, Marschner H, Hadar Y, Chen Y (1996) The role of ligand exchange in the uptake of iron from microbial siderophores by gramineous plants. Plant Physiol 112:1273–1280
Yehuda Z, Shenker M, Hadar Y, Chen Y (2000) Remedy of chlorosis induced by iron deficiency in plants with the fungal siderophore rhizoferrin. J Plant Nutr 23:1991–2006
Zamboni A, Zanin L, Tomasi N, Pezzotti M, Pinton R, Varanini Z, Cesco S (2012) Genome-wide microarray analysis of tomato roots showed defined responses to iron deficiency. BMC Genom 13:101. doi:10.1186/1471-2164-13-10
Zancan S, Cesco S, Ghisi R (2006) Effect of UV-B radiation on iron content and distribution in maize plants. Environ Exp Bot 55:266–272
Zhang FS, Römheld V, Marschner H (1991) Role of the root apoplasm for iron acquisition by wheat plants. Plant Physiol 97:1302–1305
Acknowledgments
Research was supported by grants from Italian MIUR (FIRB-Programma“ Futuro in Ricerca” and PRIN), Free University of Bolzano (TN5031 und TN5046), and Provincia Autonoma di Bolzano (Rhizotyr-TN5 218.)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tomasi, N., De Nobili, M., Gottardi, S. et al. Physiological and molecular characterization of Fe acquisition by tomato plants from natural Fe complexes. Biol Fertil Soils 49, 187–200 (2013). https://doi.org/10.1007/s00374-012-0706-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-012-0706-1