Abstract
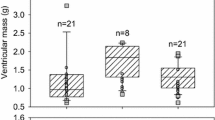
A detailed description of the electrophysiological features of cardiomyocytes in the various contractile chambers of the vertebrate heart is essential to understand the evolution of cardiac electrical activity, yet very little is known about reptiles. The present study characterizes major ionic currents (INa, ICaL, IKr, IK1 and IKACh) and action potential (AP) configuration in cardiomyocytes from the ventricle, the right atrium and the sinus venosus (SV) of Burmese pythons (Python molurus) using sharp microelectrode and patch clamp recordings. Special attention was given to SV, since it consists of myocardial cells and appears to contribute to right atrial filling in snakes. We demonstrate that most of the SV in pythons has a stable resting potential of − 82.3 ± 2.6 mV (n = 9) and lacks pacemaker activity. AP duration at 50% repolarization was similar in cells from SV and atria (350.2 ± 8.7 and 330.4 ± 17.2 ms, respectively; n = 7), but shorter than ventricular APs (557.6 ± 19.2 ms, n = 5) at 30 °C. The densities of ionic currents, however, differed substantially between atrial and SV cells, where the latter had much lower densities of INa, ICaL and IKr than atrial and ventricular myocytes. IK1 in ventricle was ninefold greater than in atrial cells and 23-fold greater than in myocytes from SV. However, IKACh was absent in ventricular cells, while it was equally large in atrial and SV myocytes. Consistent with this observation, APs of atrium and SV, but not ventricle, were greatly shortened upon addition of acetylcholine (10−6 M). Thus, snake SV, right atrium and ventricle have distinct patterns of ionic currents, but the resulting electrical activity is similar in atrium and SV.







Similar content being viewed by others
References
Abramochkin D, Kuzmin V (2018) Electrophysiological differences in cholinergic signaling between the hearts of summer and winter frogs (Rana temporaria). J Comp Physiol 188:649–656
Abramochkin DV, Vornanen M (2015) Seasonal acclimatization of the cardiac potassium currents (IK1 and IKr) in an arctic marine teleost, the navaga cod (Eleginus navaga). J Comp Physiol B 185:883–890
Abramochkin DV, Hassinen M, Vornanen M (2018) Transcripts of Kv7.1 and MinK channels and slow delayed rectifier K+ current (IKs) are expressed in zebrafish (Danio rerio) heart. Pflugers Arch 470:1753–1764
Brouillette J, Clark RB, Giles WR, Fiset C (2004) Functional properties of K+ currents in adult mouse ventricular myocytes. J Physiol 559:777–798
Davies F (1942) The conducting system of the vertebrate heart. Br Heart J. 4:66–76
Galli GLJ, Taylor EW, Shiels HA (2006) Calcium flux in turtle ventricular myocytes. Am J Physiol 291:R1781–R1789
Galli GL, Warren DE, Shiels HA (2009) Ca2+ cycling in cardiomyocytes from a high-performance reptile, the varanid lizard (Varanus exanthematicus). Am J Physiol 297:R1636–R1644
Hassinen M, Paajanen V, Haverinen J, Eronen H, Vornanen M (2007) Cloning and expression of cardiac Kir2.1 and Kir 2.2 channels in thermally acclimated rainbow trout. Am J Physiol 292:R2328–R2339
Hassinen M, Laulaja S, Paajanen V, Haverinen J, Vornanen M (2011) Thermal adaptation of the crucian carp (Carassius carassius) cardiac delayed rectifier current, IKs, by homomeric assembly of Kv7.1 subunits without MinK. Am J Physiol 301:R255–R2665
Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y (2010) Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90:291–366
Jeevaratnam K, Chadda KR, Huang CL-H, Camm AJ (2018) Cardiac potassium channels: physiological insights for targeted therapy. J Cardiovasc Pharmacol Ther 23:119–129
Jensen B, Wang T, Christoffels VM, Moorman AF (2013) Evolution and development of the building plan of the vertebrate heart. Biochim Biophys Acta 1833:783–794
Jensen B, Boukens BJ, Wang T, Moorman AF, Christoffels VM (2014a) Evolution of the sinus venosus from fish to humans. J Cardiovasc Dev Dis 1:14–28
Jensen B, Moorman AF, Wang T (2014b) Structure and function of the hearts of lizards and snakes. Biol Rev Camb Philos Soc 89:302–336
Jensen B, Vesterskov S, Boukens BJ, Nielsen JM, Moorman AFM, Christoffels VM, Wang T (2017) Morpho-functional characterization of the systemic venous pole of the reptile heart. Sci Rep 7:6644
Kleber AG, Rudy Y (2004) Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev 84:431–488
Kumari N, Gaur H, Bhargava A (2018) Cardiac voltage gated calcium channels and their regulation by β-adrenergic signaling. Life Sci 194:139–149
Li GR, Feng J, Yue L, Carrier M, Nattel S (1996) Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circ Res 78:689–696
Paajanen V, Vornanen M (2004) Regulation of action potential duration under acute heat stress by I(K, ATP) and I(K1) in fish cardiac myocytes. Am J Physiol 286:R405–R415
Panama BK, McLerie M, Lopatin AN (2007) Heterogeneity of IK1 in the mouse heart. Am J Physiol 293:H3558–3567
Sauviat MP, Colas A, Pages N (2002) Does lindane (gamma-hexachlorocyclohexane) increase the rapid delayed rectifier outward K+ current (IKr) in frog atrial myocytes? BMC Pharmacol 2:15
Savio-Galimberti E, Argenziano M, Antzelevitch C (2018) Cardiac arrhythmias related to sodium channel dysfunction. Handb Exp Pharmacol 246:331–354
Shiels HA, Vornanen M, Farrell AP (2002) The force-frequency relationship in fish hearts—a review. Comp Biochem Physiol 132A:811–826
Shih HT (1994) Anatomy of the action potential in the heart. Tex Heart Inst J 21:30–41
Stecyk JAW, Paajanen V, Farrell AP, Vornanen M (2007) Effect of temperature and prolonged anoxia exposure on electrophysiological properties of the turtle (Trachemys scripta) heart. Am J Physiol 293:R421–R437
Tamargo J, Caballero R, Gomez R, Valenzuela C, Delpon E (2004) Pharmacology of cardiac potassium channels. Cardiovasc Res 62:9–33
Varro A, Nanasi PP, Lathrop DA (1993) Potassium currents in isolated human atrial and ventricular cardiomyocytes. Acta Physiol Scand 149:133–142
Vornanen M (2016) The temperature dependence of electrical excitability in fish hearts. J Exp Biol 219:1941–1952
Vornanen M, Ryokkynen A, Nurmi A (2002) Temperature-dependent expression of sarcolemmal K(+) currents in rainbow trout atrial and ventricular myocytes. Am J Physiol 282:R1191–R1199
Vornanen M, Hassinen M, Haverinen J (2011) Tetrodotoxin sensitivity of the vertebrate cardiac Na+ current. Mar Drugs 9:2409–2422
Warren DE, Galli GLJ, Patrick SM, Shiels HA (2010) The cellular force-frequency response in ventricular myocytes from the varanid lizard, Varanus exanthematicus. Am J Physiol 298:R567–R574
Zaar M, Overgaard J, Gesser H, Wang T (2007) Contractile properties of the functionally divided python heart: two sides of the same matter. Comp Biochem Physiol 146A:163–173
Acknowledgements
The authors are grateful to Elin Petersen for excellent technical assistance and Heidi Meldgaard for animal care. The study was supported by the Russian Foundation for Basic Research [17-04-01921 to D.V.A.] and the Danish Research Council (Natur og Univers, Danmarks Frie Forskningsfond).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Additional information
Communicated by H. V. Carey.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abramochkin, D.V., Matchkov, V. & Wang, T. A characterization of the electrophysiological properties of the cardiomyocytes from ventricle, atrium and sinus venosus of the snake heart. J Comp Physiol B 190, 63–73 (2020). https://doi.org/10.1007/s00360-019-01253-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-019-01253-5