Abstract
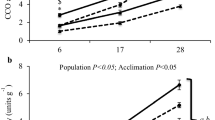
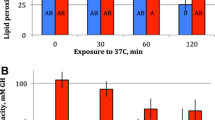
Ectothermic animals adapted to different environmental temperatures are hypothesized to have biological membranes with different chemical and physical properties such that membrane properties are optimized for their particular thermal environments. To test this hypothesis we analyzed the composition of phospholipid fatty acids (PLFAs) in seven different populations of Enchytraeus albidus originating from different thermal environments. The seven populations differ markedly in origin (polar-temperate) and are also characterized by marked difference in cold tolerance. The dominant PLFAs of E. albidus were C20:5, C20:4 and C20:2 (53–61 % of total PLFA) followed by C18:0, C20:1 and C22:2 (17–20 % of total PLFA). As hypothesized the PLFA composition varied significantly between populations and molar percentage of several of the PLFAs (particularly C18:2) correlated with the lower lethal temperature (LT50) of the seven populations. Unsaturation ratio (UFA/SFA) and average PLFA chain length also correlated significantly with LT50, such that cold sensitive populations had a shorter chain length and a lower UFA/SFA compared to cold tolerant populations. Reconstituted membranes of the least and most cold tolerant populations were used to compare membranes’ physical properties by fluorescence anisotropy and bending rigidity. Measurements of anisotropy did not show any overall difference between populations with different cold tolerance. This could be interpreted as if E. albidus populations have achieved a similar “optimal” fluidity of the membrane with a somewhat different PLFA composition. Our study suggests that membrane lipid composition could be important for the cold tolerance of E. albidus; however, these differences are not easily differentiated in the measurements of the membranes’ physical properties. Other parameters such as accumulation of glucose for cryoprotection and energy supply may also be important components of enchytraeid freeze tolerance.





Similar content being viewed by others
References
Bahrndorff S, Petersen SO, Loeschcke V, Overgaard J, Holmstrup M (2007) Differences in cold and drought tolerance of high arctic and sub-arctic populations of Megaphorura arctica Tullberg 1876 (Onychiuridae : collembola). Cryobiology 55(3):315–323
Bennett VA, Pruitt NL, Lee RE (1997) Seasonal changes in fatty acid composition associated with cold-hardening in third instar larvae of Eurosta solidaginis. J Comp Physiol B Biochem Syst Environ Physiol 167(4):249–255
Bouvrais H (2012) Bending rigidities of lipid bilayers: their determination and main inputs in biophysical studies. In: Iglic A (ed) Advances in Planar Lipid Bilayers and Liposomes, vol 15. Advances in Planar Lipid Bilayers and Liposomes. pp 1–75
Bouvrais H, Holmstrup M, Westh P, Ipsen JH (2013) Analysis of the shape fluctuations of reconstituted membranes using GUVs made from lipid extracts of invertebrates. Biol Open 2(4):373–378
Calderon S, Holmstrup M, Westh P, Overgaard J (2009) Dual roles of glucose in the freeze-tolerant earthworm Dendrobaena octaedra: cryoprotection and fuel for metabolism. J Exp Biol 212(6):859–866
Cossins AR (1977a) Adaptation of biological-membranes to temperature—effect of temperature-acclimation of goldfish upon viscosity of synaptosomal membranes. Biochim Biophys Acta 470(3):395–411
Cossins AR (1977b) Evolutionary and seasonal adaptation of membranes to temperature. Biochem Soc Trans 5(1):106–107
Cossins AR, Bowler K (1987) Temperature biology of animals. Chapman and Hall, New York
Cossins AR, Friedlander MJ, Prosser CL (1977) Correlations between behavioral temperature adaptations of goldfish and viscosity and fatty-acid composition of their synaptic-membranes. J Comp Physiol 120(2):109–121
Dimova R, Aranda S, Bezlyepkina N, Nikolov V, Riske KA, Lipowsky R (2006) A practical guide to giant vesicles. Probing the membrane nanoregime via optical microscopy. J Phys Cond Matt 18(28): S1151–S1176
Dowling NJE, Widdel F, White DC (1986) Phospholipid ester-linked fatty-acid biomarkers of acetate-oxidizing sulfate-reducers and other sulfide-forming bacteria. J Gen Microbiol 132:1815–1825
Fisker KV, Holmstrup M, Malte H, Overgaard J (2014a) Effect of repeated freeze-thaw cycles on geographically different populations of the freeze tolerant worm Enchytraeus albidus (Oligochaeta). J Exp Biol 217:3843–3852
Fisker KV, Overgaard J, Sørensen JG, Slotsbo S, Holmstrup M (2014b) Roles of carbohydrate reserves for local adaptation to low temperatures in the freeze tolerant oligochaete Enchytraeus albidus. J Comp Physiol B Biochem Syst Environ Physiol 184(2):167–177
Hayward SAL, Murray P, Gracey AY, Cossins AR (2007) Beyond the lipid hypothesis: mechanisms underlying phenotypic plasticity in inducible cold tolerance. In: Csermely P, Vigh L (eds) Molecular aspects of the stress response: Chaperones, membranes and networks. Landers Bioscience, Austin, pp 132–142
Hazel JR (1989) Cold adaptation in ectotherms: regulation of membrane function and cellular metabolism. In: Wang LCH (ed) Animal adaptation to cold. Springer, New York, pp 1–50
Hazel JR (1995) Thermal adaptation in biological-membranes—is homeoviscous adaptation the explanation. Annu Rev Physiol 57:19–42
Hazel JR, Williams EE (1990) The role of alterations in membrane lipid-composition in enabling physiological adaptation of organisms to their physical-environment. Prog Lipid Res 29(3):167–227
Henriksen J, Rowat AC, Ipsen JH (2004) Vesicle fluctuation analysis of the effects of sterols on membrane bending rigidity. Eur Biophys J Biophys Lett 33(8):732–741
Holmstrup M, Sørensen LI, Bindesbøl AM, Hedlund K (2007) Cold acclimation and lipid composition in the earthworm Dendrobaena octaedra. Comp Biochem Phys Mol Integr Physiol 147(4):911–919
Holmstrup M, Bouvrais H, Westh P, Wang C, Slotsbo S, Waagner D, Enggrob K, Ipsen JH (2014) Lipophilic contaminants influence cold tolerance of invertebrates through changes in cell membrane fluidity. Environ Sci Technol 48(16):9797–9803
Huang CH, Li S, Lin HN, Wang GQ (1996) On the bilayer phase transition temperatures for monoenoic phosphatidylcholines and phosphatidylethanolamines and the interconversion between them. Arch Biochem Biophys 334(1):135–142
Huang CH, Lin HN, Li SS, Wang GQ (1997) Influence of the positions of cis double bonds in the sn-2-acyl chain of phosphatidylethanolamine on the bilayer’s melting behavior. J Biol Chem 272(35):21917–21926
Jahnig F (1979) Structural order of lipids and proteins in membranes—evaluation of fluorescence anisotropy data. Proc Natl Acad Sci USA 76(12):6361–6365
James R, Branton D (1973) Lipid-dependent and temperature-dependent structural changes in Acholeplasma-laidlawii cell-membranes. Biochim Biophys Acta 323(3):378–390
Kates M (1986) Techniques of Lipodology. Elsevier, Amsterdam
Kostal V (2010) Cell structural modifications in insects at low temperatures. In: Denlinger DL, Lee RE (eds) Low temperature biology of insects. Cambridge University Press, Cambridge, pp 116–140
Kostal V, Simek P (1998) Changes in fatty acid composition of phospholipids and triacylglycerols after cold-acclimation of an aestivating insect prepupa. J Comp Physiol B Biochem Syst Environ Physiol 168(6):453–460
Kostal V, Berkova P, Simek P (2003) Remodelling of membrane phospholipids during transition to diapause and cold-acclimation in the larvae of Chymomyza costata (Drosophilidae). Comp Biochem Physiol Biochem Mol Biol 135(3):407–419
Lentz BR (1989) Membrane fluidity as detected by diphenylhexatriene probes. Chem Phys Lipids 50(3–4):171–190
Litman BJ, Barenholz Y (1982) Fluorescent probe: diphenylhexatriene. Methods Enzymol 81:678–685
Los DA, Murata N (2004) Membrane fluidity and its roles in the perception of environmental signals. Biochimica Et Biophysica Acta-Biomembranes 1666(1–2):142–157
Meleard P, Pott T, Bouvrais H, Ipsen JH (2011) Advantages of statistical analysis of giant vesicle flickering for bending elasticity measurements. Eur Phys J E 34(10)
Michaud MR, Denlinger DL (2006) Oleic acid is elevated in cell membranes during rapid cold-hardening and pupal diapause in the flesh fly Sarcophaga crassipalpis. J Insect Physiol 52(10):1073–1082
Mitov MD, Faucon JF, Méléard P, Bivas I, Bothorel P (1992) Thermal fluctuations of membranes. In: Gokel GW (ed) Advances In Supramolecular Chemistry. Jai Press, Greenwich, pp 93–139
Murray P, Hayward SAL, Govan GG, Gracey AY, Cossins AR (2007) An explicit test of the phospholipid saturation hypothesis of acquired cold tolerance in Caenorhabditis elegans. Proc Natl Acad Sci USA 104(13):5489–5494
Ohtsu T, Kimura MT, Katagiri C (1998) How Drosophila species acquire cold tolerance—Qualitative changes of phospholipids. Eur J Biochem 252(3):608–611
Ohtsu T, Katagiri C, Kimura MT (1999) Biochemical aspects of climatic adaptations in Drosophila curviceps, d-immigrans, and d-albomicans (Diptera : Drosophilidae). Environ Entomol 28(6):968–972
Overgaard J, Tomcala A, Sorensen JG, Holmstrup M, Krogh PH, Simek P, Kostal V (2008) Effects of acclimation temperature on thermal tolerance and membrane phospholipid composition in the fruit fly Drosophila melanogaster. J Insect Physiol 54(3):619–629
Overgaard J, Tollarova M, Hedlund K, Petersen SO, Holmstrup M (2009) Seasonal changes in lipid composition and glycogen storage associated with freeze-tolerance of the earthworm, Dendrobaena octaedra. J Comp Physiol B Biochem Syst Environ Physiol 179(5):569–577
Patrício Silva AL, Holmstrup M, Kostal V, Amorim MJB (2013) Soil salinity increases survival of freezing in the enchytraeid Enchytraeus albidus. J Exp Biol 216:2732–2740
Philip BN, Yi S-X, Elnitsky MA, Lee RE Jr (2008) Aquaporins play a role in desiccation and freeze tolerance in larvae of the goldenrod gall fly Eurosta solidaginis. J Exp Biol 211(7):1114–1119
Pott T, Bouvrais H, Méléard P (2008) Giant unilamellar vesicle formation under physiologically relevant conditions. Chem Phys Lipids 154(2):115–119
Pruitt NL, Lu C (2008) Seasonal changes in phospholipid class and class-specific fatty acid composition associated with the onset of freeze tolerance in third-instar larvae of Eurosta solidaginis. Physiol Biochem Zool 81(2):226–234
Rais A, Miller N, Stillman JH (2010) No evidence for homeoviscous adaptation in intertidal snails: analysis of membrane fluidity during thermal acclimation, thermal acclimatization, and across thermal microhabitats. Mar Biol 157(11):2407–2414
Rawicz W, Olbrich KC, McIntosh T, Needham D, Evans E (2000) Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys J 79(1):328–339
Reynolds AM, Lee RE, Costanzo JP (2014) Membrane adaptation in phospholipids and cholesterol in the widely distributed, freeze-tolerant wood frog, Rana sylvatica. J Comp Physiol B Biochem Syst Environ Physiol 184:371–383
Rozsypal J, Koštál V, Berková P, Zahradníčková H, Šimek P (2014) Seasonal changes in the composition of storage and membrane lipids in overwintering larvae of the codling moth, Cydia pomonella. J Therm Biol 45:124–133
Seelig A, Seelig J (1977) Effect of a single cis double-bond on structure of a phospholipid bilayer. Biochemistry 16(1):45–50
Shinitzky M, Barenholz Y (1978) Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta 515(4):367–394
Sinensky M (1974) Homeoviscous adaptation—homeostatic process that regulates viscosity of membrane lipids in Escherichia-coli. Proc Natl Acad Sci U S A 71(2):522–525
Singer M (1981) Permeability of phosphatidylcholine and phosphatidylethanolamine bilayers. Chem Phys Lipids 28(3):253–267
Slotsbo S, Maraldo K, Malmendal A, Nielsen NC, Holmstrup M (2008) Freeze tolerance and accumulation of cryoprotectants in the enchytraeid Enchytraeus albidus (Oligochaeta) from Greenland and Europe. Cryobiology 57(3):286–291
Stubbs CD, Smith AD (1984) The modification of mammalian membrane poly-unsaturated fatty-acid composition in relation to membrane fluidity and function. Biochim Biophys Acta 779(1):89–137
Stubbs CD, Kouyama T, Kinosita K, Ikegami A (1981) Effect of double-bonds on the dynamic properties of the hydrocarbon region of lecithin bilayers. Biochemistry 20(15):4257–4262
Tanghe A, Carbrey JM, Agre P, Thevelein JM, Van Dijck P (2005) Aquaporin expression and freeze tolerance in Candida albicans. Appl Environ Microbiol 71(10):6434–6437
Thurmond RL, Niemi AR, Lindblom G, Wieslander A, Rilfors L (1994) Membrane thickness and molecular ordering in Acholeplasma-laidlawii strain-a studied by h-2 NMR-spectroscopy. Biochemistry 33(45):13178–13188
Tomcala A, Tollarova M, Overgaard J, Simek P, Kostal V (2006) Seasonal acquisition of chill tolerance and restructuring of membrane glycerophospholipids in an overwintering insect: triggering by low temperature, desiccation and diapause progression. J Exp Biol 209(20):4102–4114
van Dooremalen C, Ellers J (2010) A moderate change in temperature induces changes in fatty acid composition of storage and membrane lipids in a soil arthropod. J Insect Physiol 56(2):178–184
Waagner D, Bouvrais H, Ipsen JH, Holmstrup M (2013a) Linking membrane physical properties and low temperature tolerance in arthropods. Cryobiology 67(3):383–385
Waagner D, Holmstrup M, Bayley M, Sørensen JG (2013b) Induced cold-tolerance mechanisms depend on duration of acclimation in the chill-sensitive Folsomia candida (Collembola). J Exp Biol 216(11):1991–2000
Acknowledgments
We thank K. L. Enggrob for assistance with measurements of cholesterol and PLFA. This study was supported by the Danish Research Council (MH), Sapere Aude DFF-Starting grants and Carlsberg foundation (JO). The stay of K·S. in Aarhus was supported by the project Postdok_BIOGLOBE (CZ.1.07/2.3.00/30.0032) co-financed by the European Social Fund and the state budget of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I.D. Hume.
Rights and permissions
About this article
Cite this article
Fisker, K.V., Bouvrais, H., Overgaard, J. et al. Membrane properties of Enchytraeus albidus originating from contrasting environments: a comparative analysis. J Comp Physiol B 185, 389–400 (2015). https://doi.org/10.1007/s00360-015-0895-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-015-0895-7