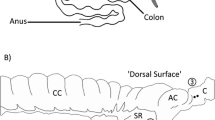
Abstract
We used high definition radial, strain rate and intensity spatiotemporal mapping to quantify contractile movements of the body and associated structures of the rabbit caecum when the terminal ileum was being perfused with saline at a constant rate. This perfusion caused gradual distension of the caecum as a result of relative restriction of outflow from the ampulla caecalis. The body of the caecum exhibited two patterns of motility that appeared autonomous, i.e. occurred independently of any contractile activity at the inlet or outlet. Firstly, the pattern that we termed ladder activity consisted of an orderly sequential contraction of bundles of axially oriented circular muscle between the spiral turns of longitudinal muscle and proceeded either from base to tip or from tip to base at a similar frequency and velocity. Secondly, less-localised, rapidly propagating synchronous contractions of both circular and longitudinal muscle, which were more common when the caecum was distended, that were termed mass peristalsis. Movements of the ileum and sacculus rotundus occurred at the same frequency and were broadly coordinated. Distension of the distal sacculus occurred synchronously with contraction of the ileum and did not propagate in an orderly manner across the structure, i.e. was instantaneous. This pattern was consistent with hydrostatic distension. Contractions propagated through the ampulla caecalis in either an orad or an aborad direction at a similar frequency to, and broadly correlated with, those in the ileum. The frequencies of distension of the sacculus and of contraction in the ileum and ampulla were momentarily augmented during mass peristalsis. The authors conclude that there was some coordination between the contractile activity of the terminal ileum and the caecal ampulla during periods of ongoing inflow from the ileum and between these structures and the caecum during mass peristalsis.








Similar content being viewed by others
References
Besoluk K, Eken E, Sur E (2006) A morphological and morphometrical study on the sacculus rotundus and ileum of the Angora rabbit. Vet Med (Praha) 51:60–65
Björnhag G (1972) Separation and delay of contents in the rabbit colon. Swed J Agric Res 2:125–136
Björnhag G (1981) Separation and retrograde transport in the large intestine of herbivores. Livest Prod Sci 8:351–360
Björnhag G (1987) Comparative aspects of digestion in the hindgut of mammals. The colonic separator mechanism (CSM) (a review). Dtsch Tierarztl Wochenschr 94:33–36
Ehrlein HJ, Ruoff G (1982) Cecal motility and flow of ingesta from the ileum to the cecum, appendix, and colon in rabbits. In: Wienbeck M (ed) Motility of the digestive tract. Raven, New York, pp 475–481
Fioramonti J, Ruckebusch Y (1974) La motricité caecale chez le lapin. I. Nature des contractions. Ann Rech Vétér 5:1–13
Hennig GW, Costa M, Chen BN, Brookes SJH (1999) Quantitative analysis of peristalsis in the guinea-pig small intestine using spatio-temporal maps. J Physiol 517:575–590
Janssen PWM, Lentle RG, Hulls C, Ravindran V, Amerah AM (2009) Spatiotemporal mapping of the motility of the isolated chicken caecum. J Comp Physiol B179:593–604
Lentle RG, Janssen PWM, Asvarujanon P, Chambers P, Stafford KJ, Hemar Y (2007) High definition mapping of circular and longitudinal motility in the terminal ileum of the brushtail possum Trichosurus vulpecula with watery and viscous perfusates. J Comp Physiol B177:543–556
Lentle RG, Janssen PWM, Asvarujanon P, Chambers P, Stafford KJ, Hemar Y (2008) High definition spatiotemporal mapping of contractile activity in the isolated proximal colon of the rabbit. J Comp Physiol B178:257–268
Lentle RG, Janssen PWM, Hume ID (2009) The roles of filtration and expression in the processing of digesta with high solid phase content. Comp Biochem Physiol A 154:1–9
Pallotta N, Cicala M, Frandina C, Corazziari E (1998) Antro-pyloric contractile patterns and transpyloric flow after meal ingestion in humans. Am J Gastroenterol 93:2513–2522
Ruckebusch Y, Hörnicke H (1977) Motility of the rabbit’s colon and cecotrophy. Physiol Behav 18:871–878
Schulze-Delrieu K, Brown BP, Lange W, Custer-Hagen T, Lu C, Shirazi S, Lepsien G (1996) Volume shifts, unfolding and rolling of haustra in the isolated guinea pig caecum. Neurogastroenterol Mot 8:217–225
Snipes RL (1978) Anatomy of the rabbit cecum. Anat Embryol (Berl) 155:57–80
Snipes R (1982) Anatomy of the guinea-pig cecum. Anat Embryol (Berl) 165:97
Stevens CE, Hume ID (1995) Comparative physiology of the vertebrate digestive system. Cambridge University Press, New York
Trendelenburg P (1917) Physiologische und pharmakologische Versuche über die Dünndarmperistaltik. Naunyn Schmiedebergs Arch Pharmacol 81:55–129
Van Soest PJ (1994) Nutritional Ecology of the Ruminant. Cornell University Press, Ithaca
Wang XY, Lammers W, Bercik P, Huizinga JD (2005) Lack of pyloric interstitial cells of Cajal explains distinct peristaltic motor patterns in stomach and small intestine. Am J Physiol 289:G539–G549
Warner ACI (1981) Rate of passage of digesta through the gut of mammals and birds. Nutr Abstr Rev 51:789–820
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I.D. Hume.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MPG 1536 kb)
Rights and permissions
About this article
Cite this article
Hulls, C., Lentle, R.G., de Loubens, C. et al. Spatiotemporal mapping of ex vivo motility in the caecum of the rabbit. J Comp Physiol B 182, 287–297 (2012). https://doi.org/10.1007/s00360-011-0610-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-011-0610-2