Abstract
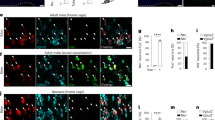
Location of the lung respiratory rhythm generator (RRG) in the bullfrog brainstem was investigated by examining neurokinin-1 and μ-opioid receptor (NK1R, μOR) colocalization by immunohistochemistry and characterizing the role of these receptors in lung rhythm and episodic pattern generation. NK1R and μOR occurred in brainstems from all developmental stages. In juvenile bullfrogs a distinct area of colocalization was coincident with high-intensity fluorescent labeling of μOR; high-intensity labeling of μOR was not distinctly and consistently localized in tadpole brainstems. NK1R labeling intensity did not change with development. Similarity in colocalization is consistent with similarity in responses to substance P (SP, NK1R agonist) and DAMGO (μOR agonist) when bath applied to bullfrog brainstems of different developmental stages. In early stage tadpoles and juvenile bullfrogs, SP increased and DAMGO decreased lung burst frequency. In juvenile bullfrogs, SP increased lung burst frequency, episode frequency, but decreased number of lung bursts per episode and lung burst duration. In contrast, DAMGO decreased lung burst frequency and burst cycle frequency, episode frequency, and number of lung bursts per episode but increased all other lung burst parameters. Based on these results, we hypothesize that NK1R and μOR colocalization together with a metamorphosis-related increase in μOR intensity marks the location of the lung RRG but not necessarily the lung episodic pattern generator.







Similar content being viewed by others
References
Adli DSH, Steusse SL, Cruce WLR (1999) Immunohistochemistry and spinal projections of the reticular formation in the northern leopard frog, Rana pipiens. J Comp Neurol 404:387–407
Bayliss D, Viana F, Berger A (1992) Mechanisms underlying excitatory effects of thyrotropin-releasing hormone on rat hypoglossal motoneurons in vitro. J Neurophysiol 68:1733–1745
Belzile O, Gulemetova R, Kinkead R (2002) Role of 5HT2A/C receptors in serotonergic modulation of respiratory motor output during tadpole development. Respir Physiol Neurobiol 133:277–282
Chen A, Hedrick M (2008) Role of glutamate and substance P in the amphibian respiratory network during development. Respir Physiol Neurobiol. doi:10-1016/j.resp.2008.03.010
Chen Z, Hedner T, Hedner J (1990) Local application of somatostatin in the rat ventrolateral brain medulla induces apnea. J Appl Physiol 69:2233–2238
Dong X, Feldman J (1995) Modulation of inspiratory drive to phrenic motoneurons by presynaptic adenosine A1 receptors. J Neurosci 15:3458–3467
Folbergrova J, Norberg K, Quistorff B, Siesjo B (1975) Carbohydrate and amino acid metabolism in rat cerebral cortex in moderate and extreme hypercapnia. J Neurochem 25:457–462
Galante R, Kubin L, Fishman A, Pack A (1996) Role of chloride-mediated inhibition in respiratory rhythmogenesis in an in vitro brainstem of tadpole, Rana catesbeiana. J Physiol 492:545–558
Gdovin M, Torgerson C, Remmers J (1996) Characterization of gill and lung ventilatory activity in cranial nerves in the spontaneously breathing tadpole, Rana catesbeiana. FASEB J 10:A642
Gray P, Rekling J, Bocchiaro C, Feldman J (1999) Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science 286:1566–1568
Gray P, Janczewski W, Mellen N, McCrimmon D, Feldman J (2001) Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci 4:927–930
Guyenet P, Mulkey D, Stornetta R, Bayliss D (2005) Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci 25:8938–8947
Harris M, Wilson R, Vasilakos K, Taylor B, Remmers J (2002) Central respiratory activity of the tadpole in vitro brain stem is modulated diversely by nitric oxide. Am J Regulatory Int Comp Physiol 283:R417–R428
Jackson D (1978) Respiratory control in air breathing ectotherms. In: Davies D, Barnes C (eds) Regulation of ventilation and gas exchange. Academic Press, Dublin, pp 93–130
Kazemi H, Hoop B (1991) Glutamic acid and gamma-aminobutyric acid neurotransmitters in central control of breathing. J Appl Physiol 70:1–7
Kinkead R (1997) Episodic breathing in frogs: converging hypotheses on neural control of respiration in air breathing vertebrates. Amer Zool 37:31–40
Kinkead R, Milsom W (1994) Chemoreceptors and control of episodic breathing in the bullfrog (Rana catesbeiana). Respir Physiol 95:81–98
Kinkead R, Milsom W (1996) CO2-sensitive olfactory and pulmonary receptor modulation of episodic breathing in bullfrogs. Am J Physiol 270:R134–R144
Kinkead R, Milsom W (1997) Role of pulmonary stretch receptor feedback in the control of episodic breathing in the bullfrog. Am J Physiol 272:R497–R508
Kinkead R, Filmyer W, Mitchell G, Milsom W (1994) Vagal input enhances responsiveness of respiratory discharge to central changes in pH/CO2 in bullfrogs. J Appl Physiol 77:2048–2051
Liu Y, Wong-Riley M, Liu J, Wei X, Jia Y, Liu H, Fujiyama F, Ju G (2004) Substance P and enkephalinergic synapses onto neurokinin-1 receptor-immunoreactive neurons in the pre-Bötzinger complex of rats. Eur J Neurosci 19:65–75
Llona I, Ampuero E, Eugenin J (2004) Somatostatin inhibition of fictive respiration is modulated by pH. Brain Res 1026:136–142
Maggi C, Patacchini R, Rovero P, Giachetti A (1993) Tachykinin receptors and tachykinin receptor antagonists. J Auton Pharmacol 13:23–93
Mantyh P, DeMaster E, Malhotra A, Ghilardi J, Rogers S et al (1995) Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science 268:1629–1632
Mantyh P, Rogers D, Monroe P, Allen B, Rea Ghilardi (1997) Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science 278:275–279
McAneney J, Reid S (2007) Chronic hypoxia attenuates central respiratory-related pH/CO2 chemosensitivity in the cane toad. Resp Physiol Neurobiol 156:266–275
Mellen N, Janczewski W, Bocchiaro C, Feldman J (2003) Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron 37:821–826
Metz B (1966) Hypercapnia and acetylcholine release from the cortex and medulla. J Physiol Lond 186:321–322
Nattie E, Li A (2002) Substance P-saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J Physiol 544:603–616
Nattie E, Prabhakar N (2001) Peripheral and central chemosensitivity: multiple mechanisms, multiple sites? A workshop summary. Adv Exp Med Biol 499:73–80
Onimaru H, Arata A, Homma I (1995) Intrinsic burst generation in pre-inspiratory neurons in the medulla of brainstem-spinal cord preparations isolated from newborn rats. Exp Brain Res 106:57–68
Onimaru H, Arata A, Homma I (1997) Neuronal mechanisms of respiratory rhythm generation; an approach using in vitro preparation. Jpn J Physiol 47:385–403
Ptak K, Di Pasquale E, Monteau R (1999) Substance P and central respiratory activity: a comparative in vitro study on fetal and newborn rat. Brain Res Dev Brain Res 114:217–227
Rekling J (1990) Excitatory effects of thyrotropin-releasing hormone (TRH) in hypoglossal motoneurons. Brain Res 510:175–179
Rekling J, Feldman J (1998) PreBötzinger Complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol 60:385–405
Smatresk N, Smits A (1991) Effects of central and peripheral chemoreceptor stimulation on ventilation in the marine toad, Bufo marinus. Respir Physiol 83:223–238
Smith J, Ellenberger H, Ballanyi K, Richter D, Feldman J (1991) Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254:726–729
Srinivasan M, Goiny M, Pantaleo T et al (1991) Enhanced in vivo release of substance P in the nucleus tractus solitarius during hypoxia in the rabbit: role of peripheral input. Brain Res 546:211–216
Straus C, Wilson R, Remmers J (2000a) Developmental disinhibition: turning off inhibition turns on breathing in vertebrates. J Neurobiol 45:75–83
Straus C, Wilson R, Tezenas du Montcel S, Remmers J (2000b) Baclofen eliminated cluster lung breathing of the tadpole brainstem, in vitro. Neurosci Lett 292:13–16
Stuesse S, Adli D, Cruce W (2001) Immunochemical distribution of enkephalin, substance P and somatostatin in the brainstem of the leopard frog, Rana pipiens. Microsc Res Tech 54:229–245
Takeda S, Eriksson L, Yamamoto Y, Joensen H, Onimaru H, Lindahl S (2001) Opioid action on respiratory neuron activity of the isolated respiratory network in newborn rats. Anesthesiology 95:740–749
Taylor A, Köllros J (1946) Stages in the normal development of Rana pipiens larvae. Anat Rec 94:7–24
Taylor B, Harris M, Leiter J, Gdovin M (2003) Ontogeny of central CO2 chemoreception: chemosensitivity in the ventral medulla of developing bullfrogs. Am J Physiol Regul Integr Comp Physiol 285:R1461–R1472
Telgkamp P, Cao Y, Basbaum A, Ramirez J (2002) Long-term deprivation of substance P in PPT-A mutant mice alters the anoxic response of the isolated respiratory network. J Neurophysiol 88:206–213
Torgerson C, Gdovin M, Remmers J (1997) Ontogeny of central chemoreception during fictive gill and lung ventilation in an in vitro brainstem preparation of Rana catesbeiana. J Exp Biol 200:2063–2072
Vasilakos K, Wilson R, Kimura N, Remmers J (2005) Ancient gill and lung oscillators may generate the respiratory rhythm of frogs and rats. J Neurobiol 62:369–385
Wang H, Germanson T, Guyenet P (2002) Depressor and tachypneic responses to chemical stimulation of the ventral respiratory group are reduced by ablation of neurokinin-1 receptor-expressing neurons. J Neurosci 22:3755–3764
West N, Topor Z, van Vliet B (1987) Hypoxemic threshold for lung ventilation in the toad. Respir Physiol 70:377–390
Weyne J, Leuven F, Kazemi H, Leusen I (1978) Selected brain amino acids and ammonium during chronic hypercapnia in conscious rats. J Appl Physiol 44:333–339
Wilson R, Vasilakos K, Harris M, Straus C, Remmers J (2002) Evidence that ventilatory rhythmogenesis in the frog involves two distinct neuronal oscillators. J Physiol 540:557–570
Wilson R, Vasilakos K, Remmers J (2006) Phylogeny of vertebrate respiratory rhythm generators: the oscillator homology hypothesis. Resp Physiol and Neurobiol 154:47–60
Wong-Riley M, Liu Q (2005) Neurochemical development of brain stem nuclei involved in the control of respiration. Resp Physiol and Neurobiol 149:83–98
Yamamoto Y, Onimaru H, Homma I (1992) Effect of substance P on respiratory rhythm and pre-inspiratory neurons in the ventrolateral structure of rostral medulla oblongata: an in vivo study. Brain Res 599:272–278
Acknowledgments
This work was funded by NIH-NINDS 2U54NS041069-06AI. Protocols used in this study follow the institutional animal care and use committee (IACUC) guidelines and adhere to local and national ethical standards.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. V. Carey.
Rights and permissions
About this article
Cite this article
Davies, B.L., Brundage, C.M., Harris, M.B. et al. Lung respiratory rhythm and pattern generation in the bullfrog: role of neurokinin-1 and μ-opioid receptors. J Comp Physiol B 179, 579–592 (2009). https://doi.org/10.1007/s00360-009-0339-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-009-0339-3