Abstract
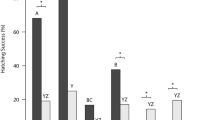
Many reptiles lay eggs with flexible shells that can progressively lose water until lethal dehydration under dry soil conditions. The number of eggs that develop together may influence the water exchange in the nest. We hypothesise that egg aggregation could reduce water lost under dry conditions. We exposed aggregated and isolated eggs to severe hydric stress followed by a period of rehydration. Hydric stress caused a general loss of water in common chameleon eggs. Initial egg mass did not affect survival but eggs that had lost more water had higher mortality and produced smaller hatchlings. Mass loss was higher and even lethal for isolated Chamaeleo chameleon eggs. However, aggregated eggs lost less water and most survived this period. After hydric stress, all surviving eggs gained mass via water absorption, and aggregation negatively affected water uptake. Isolated eggs hatched at smaller sizes than aggregated eggs. Aggregation also favoured hatching synchrony. Large clutches may favour hatching success of terrestrial flexible-shelled eggs incubated under severe drought conditions.





Similar content being viewed by others
References
Ackerman RA (1991) Physical factors affecting the water exchange of buried reptile eggs. In: Deeming DC, Ferguson MWK (eds) Egg incubation: its effects on embryonic development in birds and reptiles. Cambridge University Press, Cambridge, pp 193–212
Andrews RM, Díaz-Paniagua C, Marco A, Portheault A (2008) Developmental arrest during embryonic development of the common chameleon (Chamaeleo chamaeleon) in Spain. Physiol Biochem Zool (in press)
Andrews RM, Donoghue S (2004) Effects of temperature and moisture on embryonic diapause of the Veiled Chameleon (Chamaeleo calyptratus). J Exp Zool 301A:629–635
Belinsky A, Ackerman RA, Dmi’el R, Ar A (2004) Water in reptilian eggs and hatchlings. In: Deeming DC (ed) Reptilian incubation; environment, evolution and behaviour. Nottingham University Press, Nottingham, pp 125–141
Black CP, Birchard GF, Schuett GW, Black VD (1984) Influence of incubation substrate water content on oxygen uptake in embryos of the Burmese python (Python molurus bioittatus). In: Seymour RS (ed) Respiration and metabolism of embryonic vertebrates. Dr. W Junk, Dordrecht, pp 137–146
Blasco M, Cano J, Crespillo E, Escudero JC, Romero J, Sánchez M (1985) El Camaleón Común (Chamaeleo chamaeleon) en la Península Ibérica. ICONA-Ministerio Agricultura, Pesca y Alimentación, Madrid
Blázquez MC, Díaz-Paniagua C, Mateo JA (2000) Egg retention and mortality of gravid and nesting female chameleons in southern Spain. Herpetol J 10:91–94
Bons J, Bons N (1960) Notes sur la reproduction et le développement de Chamaeleo chamaeleon (L.). Bull Soc Sci Nat Phys Maroc 40:323–335
Díaz-Paniagua C (2007) Effect of cold temperature on the length of incubation of Chamaeleo chamaeleon. Amphib-Reptil 28:1–6
Díaz-Paniagua C, Cuadrado M (2003) Influence of incubation conditions on hatching success, embryo development and hatchling phenotype of common chameleon (Chamaeleo chamaeleon) eggs. Amphib-Reptil 24:429–440
Díaz-Paniagua C, Cuadrado M, Blázquez MC, Mateo JA (2002) Reproduction of Chamaeleo chamaeleon under contrasting environmental conditions. Herpetol J 12:99–104
Finkler MS (1999) Influence of water availability during incubation on hatchling size, body composition, desiccation tolerance, and terrestrial locomotor performance in the snapping turtle Chelydra serpentina. Physiol Biochem Zool 72:714–722
Gutzke WHN, Packard GC (1987) Influence of the hydric and thermal environments on eggs and hatchlings of bull snakes Pituophis melanoleucus. Physiol Zool 60:9–17
Gutzke WHN, Packard GC, Packard MJ, Boardman TJ (1987) Influence of the hydric and thermal environments on eggs and hatchlings of painted turtles (Chrysemys picta). Herpetologica 43:393–404
Janzen FJ, Ast JC, Paukstis GL (1995) Influence of the hydric environment and clutch on eggs and embryos of two sympatric map turtles. Funct Ecol 9:913–922
Ji X, Du WG (2001) The effects of thermal and hydric environments on hatching success, embryonic use of energy and hatchling traits in a colubrid snake, Elaphe carinata. Comp Biochem Physiol A 129:461–471
Marco A, Díaz-Paniagua C, Hidalgo-Vila J (2004) Influence of egg aggregation and soil moisture on incubation of flexible-shelled lacertid lizard eggs. Can J Zool 82:60–65
Muth A (1980) Physiological ecology of the desert iguana (Dipsosaurus dorsalis) eggs: temperature and water relations. Ecology 61:1335–1343
Overall KL (1994) Lizard egg environments. In: Vitt LJ, Pianka ER (eds) Lizard ecology: historical and experimental perspectives. Princeton University Press, New Jersey, pp 51–72
Packard GC (1991) Physiological and ecological importance of water to embryos of oviparous reptiles. In: Deeming DC, Ferguson MWK (eds) Egg incubation: its effects on embryonic development in birds and reptiles. Cambridge University Press, Cambridge, pp 213–228
Packard GC (1999) Water relations of chelonian eggs and embryos: is wetter better? Am Zool 39:289–303
Packard GC, Packard MJ (1988) The physiological ecology of reptilian eggs and embryos. In: Gans C, Huey RB (eds) Biology of the reptilia, vol 16-ecology B, defense and life history. C Alan R Liss Inc, New York, pp 523–606
Spencer R-J, Thompson MB, Banks PB (2001) Hatch or wait? A dilemma in reptilian incubation. Oikos 93:401–406
Thompson MB (1987) Water exchange in reptilian eggs. Physiol Zool 60:1–8
Tracy CR, Snell KL (1985) Interrelations among water and energy relations of reptilian eggs, embryos, and hatchlings. Am Zool 25:999–1008
Vleck D (1991) Water economy and solute regulation of reptilian and avian embryos. In: Deeming DE, Ferguson MWJ (eds) Egg incubation: its effects on embryonic development in birds and reptiles. Cambridge University Press, Cambridge, pp 245–259
Wiewandt TA (1982) Evolution of nesting patterns in Iguanine lizards. In: Burghardt GM, Rand AS (eds) Iguanas of the world. Noyes Publisher, New Jersey, pp 119–141
Acknowledgments
Thanks to Ana Andreu, Juan José Gómez, Antonio Conejo and Wouter de Vries for their help and to Robin Andrews for editorial assistance. The Centro de Recuperación de Especies Amenazadas de El Puerto de Santa María allowed us to use their facilities to incubate chameleon eggs under natural conditions. The Spanish Ministry of Science and Technology funded this study (CICYT PB97-1162). The study was conducted with the permission of the Consejería de Medio Ambiente, Junta de Andalucía, Spain and complies with current Spanish legislation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.V. Carey.
Rights and permissions
About this article
Cite this article
Marco, A., Díaz-Paniagua, C. Aggregation protects flexible-shelled reptile eggs from severe hydric stress. J Comp Physiol B 178, 421–428 (2008). https://doi.org/10.1007/s00360-007-0234-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-007-0234-8