Abstract
Introduction and objective
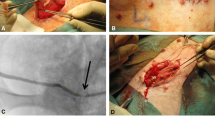
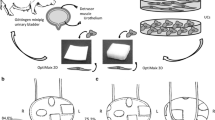
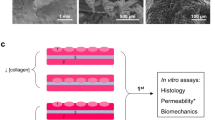
Tissue-engineered materials in urethral reconstructive surgeries are a promising field for innovative therapy. Collagen matrices increase stability of cell-based implants and can promote viability and proliferation of urothelial cells. In this study, a collagen type I-based cell carrier (CCC) with stratified multi-layer autologous urothelium was used for urethroplasty after induction of urethral stricture in eight minipigs.
Materials and methods
Minipigs underwent surgical procedures to induce urethral stricture by thermocoagulation. Simultaneously, bladder tissue was harvested. Urothelial cells were expanded, labeled with PKH26 and seeded onto CCC in high density. 3 weeks after strictures were induced and verified by urethrography, minipigs underwent urethroplasty using the seeded CCC. Two animals were euthanized after 1, 2, 4, and 24 weeks. Urethras were histologically examined for integration and survival of seeded CCC. In vivo phenotype of multi-layered urothelium matrix constructs was characterized via immunofluorescence staining with pancytokeratin, CK20, p63, E-cadherin and ZO-1.
Results
Seeded CCCs showed excellent stability and suturability after manipulation and application. Transplanted cells were detected using positive PKH26 fluorescence up to 6 months after labeling. Urothelium matrix implants integrated well into the host tissue without sign of inflammation. Animals showed no sign of rejection or stricture recurrence (urethrography) at any time during experimental period. Immunofluorescence analysis confirmed epithelial phenotype, junction formation and differentiation after 2 weeks.
Conclusion
CCC can be suitable for urologic reconstructive surgeries and represents a promising option for clinical application. Longer follow-up results are required to exclude re-occurrence of stricture reformation.





Similar content being viewed by others
References
Versteegden LR et al (2017) Tissue engineering of the urethra: a systematic review and meta-analysis of preclinical and clinical studies. Eur Urol. https://doi.org/10.1016/j.eururo.2017.03.026
Chapple C et al (2014) SIU/ICUD Consultation on urethral strictures: the management of anterior urethral stricture disease using substitution urethroplasty. Urology 83:S31–S47. https://doi.org/10.1016/j.urology.2013.09.012
Damaser MS, Sievert KD (2019) Reviving regenerative urology. Nat Rev Urol. 16(3):143–144. https://doi.org/10.1038/s41585-018-0131-9
Lumen N, Oosterlinck W, Hoebeke P (2012) Urethral reconstruction using buccal mucosa or penile skin grafts: systematic review and meta-analysis. Urol Int 89:387–394. https://doi.org/10.1159/000341138
Dubey D et al (2007) Dorsal onlay buccal mucosa versus penile skin flap urethroplasty for anterior urethral strictures: results from a randomized prospective trial. J Urol 178:2466–2469. https://doi.org/10.1016/j.juro.2007.08.010
Sievert K-D (2017) Tissue engineering of the urethra: solid basic research and farsighted planning are required for clinical application. Eur Urol 52:1580–1589. https://doi.org/10.1015/eururo.2017.04.025
Mundy A (1995) The long-term results of skin inlay urethroplasty. Br J Urol 75:59–61
Orabi H et al (2013) Tissue engineering of urinary bladder and urethra: advances from bench to patients. Sci World J 2013:154564. https://doi.org/10.1155/2013/154564
Tian H et al (2010) Differentiation of human bone marrow mesenchymal stem cells into bladder cells: potential for urological tissue engineering. Tissue Eng A. 16(5):1769–1779
Ning J et al (2011) Bone marrow mesenchymal stem cells differentiate into urothelial cells and the implications for reconstructing urinary bladder mucosa. Cytotechnology 63(5):531–539
Zhao Z et al (2016) Differentiate into urothelium and smooth muscle cells from adipose tissue-derived stem cells for ureter reconstruction in a rabbit model. Am J Transl Res. 8(9):3757–3768
Oottamasathien S et al (2007) Directed differentiation of embryonic stem cells into bladder tissue. Dev Biol. 304(2):556–566
Kang M, Kim H, Han Y (2014) Generation of bladder urothelium from human pluripotent stem cells under chemically defined serum- and feeder-free system. Int J Mol Sci 15(5):7139–7157
Feil G et al (2008) Immunoreactivity of p63 in monolayered and in vitro stratified human urothelial cell cultures compared with native urothelial tissue. Eur Urol 53(5):1066–1072
Schmidt T et al (2011) Evaluation of a thin and mechanically stable collagen cell carrier. Tissue Eng Part C Methods 17(12):1161–1170
Held M et al (2016) Biomechanical skin property evaluation for wounds treated with synthetic and biosynthetic wound dressings and a newly developed collagen matrix during healing of superficial skin defects in a rat models. Wounds 28(9):334–340 (PMID 27701129)
Seibold J et al (2012) Development of a porcine animal model for urethral stricture repair using autologous urothelial cells. J Pediatr Urol 8:194–200. https://doi.org/10.1016/j.jpurol.2011.02.001
Sievert K-D et al (2012) Introducing a large animal model to create urethral stricture similar to human stricture disease: a comparative experimental microscopic study. J Urol 187(3):1101–1109
Bhargava S, Chapple CR (2004) Buccal mucosal urethroplasty: is it the new gold standard? BJU Int 93(9):1191–1193
XiangGuo L et al (2018) A smart bilayered scaffold supporting keratinocytes and muscle cells in micro/nano-scale for urethral reconstruction. Theranostics. 8(15):4152–4154
Palminteri E et al (2012) Long-term results of small intestinal submucosa graft in bulbar urethral reconstruction. Urology 79(3):695–701
Liu Y et al (2017) Urethral reconstruction with autologous urine-derived stem cells seeded in three-dimensional porous small intestinal submucosa in a rabbit model. Stem Cell Res Ther 8:63. https://doi.org/10.1186/s13287-017-0500-y
Orabi H et al (2013) Cell-seeded tubularized scaffolds for reconstruction of long urethral defects: a preclinical study. Eur Urol 63(3):531–538
Raya-Rivera A et al (2011) Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet 377(9772):1175–1182
Bhargava S et al (2008) Tissue-engineered buccal mucosa urethroplasty-clinical outcomes. Eur Urol 53(6):1263–1269
Feil G et al (2008) Bioartifizielles autologes Urothel etabliert aus Spülungen der Harnblase. Urologe 47:1091–1096. https://doi.org/10.1007/s00120-008-1849-4
Seibold J et al (2011) Development of a porcine animal model for urethral stricture repair using autologous urothelial cells. J Pediatr Urol. 8(2):194–200
Vaegler M et al (2016) A bovine collagen type i-based biodegradable matrix as a carrier for tissue-engineered urothelium. J Stem Cell Res Ther. 5:275. https://doi.org/10.4172/2157-7633.1000275
Aufderklamm S et al (2017) Collagen cell carriers seeded with human urothelial cells for urethral reconstructive surgery: first results in a xenograft minipig model. World J Urol 35(7):1125–1132. https://doi.org/10.1007/s00345-016-1959-3
Daum L, Maurer S, Vaegler M, Sievert KD (2015) In vivo biocompatibility of a collagen cell carrier seeded with human urothelial cells in Rats. J Cell Sci Ther. 6:215. https://doi.org/10.4172/2157-7013.1000215
Dehoux JP, Gianello P (2007) The importance of large animal models in transplantation. Front Biosci. 12:4864–4880
Sievert K-D, Selent-Stier C, Wiedemann J et al (2012) Introducing a large animal model to create urethral stricture similar to human stricture disease: a comparative experimental microscopic study. J Urol 187:1101–1109. https://doi.org/10.1016/j.juro.2011.10.132
Acknowledgements
The study was supported by Viscofan BioEngineering Germany (CU2/12). The authors would also like to thank Kathyrn Emily Nelson of KNM Communications for her editorial assistance.
Author information
Authors and Affiliations
Contributions
KDS: project development, supervision, analysis and supervision of data, manuscript writing, critical revision of manuscript; LD: data collection and manuscript drafting; SM: data collection; PT: manuscript writing/editing; MV: data collection/management, analysis and supervision of data, manuscript drafting; SA: data collection/management, analysis and supervision of data, critical revision of manuscript; BA: analysis and supervision of data, analysis and supervision of data, critical revision of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author declare that there no conflicts of interests.
Statement of welfare of animals
All applicable, international, national and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sievert, KD., Daum, L., Maurer, S. et al. Urethroplasty performed with an autologous urothelium-vegetated collagen fleece to treat urethral stricture in the minipig model. World J Urol 38, 2123–2131 (2020). https://doi.org/10.1007/s00345-019-02888-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-019-02888-3