Abstract
Objectives
To evaluate the discrimination, calibration, and net benefit performance of the Prostate Cancer Prevention Trial Risk Calculator (PCPTRC) across five European randomized study of screening for prostate cancer (ERSPC), 1 United Kingdom, 1 Austrian, and 3 US biopsy cohorts.
Methods
PCPTRC risks were calculated for 25,733 biopsies using prostate-specific antigen (PSA), digital rectal examination, family history, history of prior biopsy, and imputation for missing covariates. Predictions were evaluated using the areas underneath the receiver operating characteristic curves (AUC), discrimination slopes, chi-square tests of goodness of fit, and net benefit decision curves.
Results
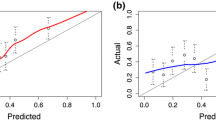
AUCs of the PCPTRC ranged from a low of 56% in the ERSPC Goeteborg Rounds 2–6 cohort to a high of 72% in the ERSPC Goeteborg Round 1 cohort and were statistically significantly higher than that of PSA in 6 out of the 10 cohorts. The PCPTRC was well calibrated in the SABOR, Tyrol, and Durham cohorts. There was limited to no net benefit to using the PCPTRC for biopsy referral compared to biopsying all or no men in all five ERSPC cohorts and benefit within a limited range of risk thresholds in all other cohorts.
Conclusions
External validation of the PCPTRC across ten cohorts revealed varying degree of success highly dependent on the cohort, most likely due to different criteria for and work-up before biopsy. Future validation studies of new calculators for prostate cancer should acknowledge the potential impact of the specific cohort studied when reporting successful versus failed validation.

Similar content being viewed by others
References
Thompson IM, Ankerst DP, Chi C, Goodman PJ, Tangen CM, Lucia MS, Feng Z, Parnes HL, Coltman CA Jr (2006) Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 98:529–534
Vickers AJ, Cronin AM, Roobol MJ, Hugosson J, Jones JS, Kattan MW, Klein E, Hamdy F, Neal D, Donovan J, Parekh DJ, Ankerst D, Bartsch G, Klocker H, Horninger W, Benchikh A, Salama G, Villers A, Freedland SJ, Moreira DM, Schroeder FH, Lilja H (2010) The relationship between prostate-specific antigen and prostate cancer risk: the Prostate Biopsy Collaborative Group. Clin Cancer Res 16:4374–4381
van Buuren S (2007) Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 16:219–242
Janssen KJM, Donders ART, Harrell FE Jr, Vergouwe Y, Chen Q, Grobbee DE, Moons KGM (2010) Missing covariate data in medical research: to impute is better than to ignore. J Clin Epidemiol 63:721–727
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Steyerberg EW (2010) Clinical prediction models. Springer, New York, pp 270–271
Vickers AJ, Elkin EB (2006) Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 26:565–574
Cavadas V, Osório L, Sabell F, Teves F, Branco F, Silva-Ramos M (2010) Prostate cancer prevention trial and European randomized study of screening for prostate cancer risk calculators: a performance comparison in a contemporary screened cohort. Eur Urol 58:551–558
Eyre SJ, Ankerst DP, Wei JT, Nair PV, Regan MM, Bueti G, Tang J, Rubin MA, Kearney M, Thompson IM, Sanda MG (2009) Validation in a multiple urology practice setting of the prostate cancer prevention trial calculator for predicting prostate cancer detection. J Urol 182:2653–2658
Hernandez DJ, Han M, Humphreys EB, Mangold LA, Taneja SS, Childs SJ, Bartsch G, Partin AW (2009) Predicting the outcome of prostate biopsy: comparison of a novel logistic regression-based model, the prostate cancer risk calculator, and prostate-specific antigen level alone. BJU Int 103:609–614
Nguyen CT, Yu C, Moussa A, Kattan MW, Jones JS (2010) Performance of prostate cancer prevention trial risk calculator in a contemporary cohort screened for prostate cancer and diagnosed by extended prostate biopsy. J Urol 183:529–533
Oliveira M, Marques V, Carvalho AP, Santos A (2011) Head-to-head comparison of two online nomograms for prostate biopsy outcome prediction. BJU Int 107:1780–1783
Parekh DJ, Ankerst DP, Higgins BA, Hernandez J, Canby-Hagino E, Brand T, Troyer DA, Leach RJ, Thompson IM (2006) External validation of the prostate cancer prevention trial risk calculator in a screened population. Urology 68:1153–1155
van den Bergh RC, Roobol MJ, Wolters T, van Leeuwen PJ, Schroder FH (2008) The prostate cancer prevention trial and European randomized study of screening for prostate cancer risk calculators indicating a positive prostate biopsy: a comparison. BJU Int 102:1068–1073
Ankerst DP, Groskopf J, Day JR, Blase A, Rittenhouse H, Pollock BH, Tangen C, Parekh D, Leach RJ, Thompson I (2008) Predicting prostate cancer risk through incorporation of prostate cancer gene 3. J Urol 180:1303–1308
Prior C, Guillen-Grima F, Robles JE, Rosell D, Fernandez-Montero JM, Agirre X, Catena R, Calvo A (2010) Use of a combination of biomarkers in serum and urine to improve detection of prostate cancer. World J Urol 28:681–686
Vickers AJ, Cronin AM (2010) Everything you always wanted to know about evaluating prediction models (but were too afraid to ask). Urology 76:1298–1301
Kattan MW (2011) Factors affecting the accuracy of prediction models limit the comparison of rival prediction models when applied to separate data sets. Eur Urol 59:566–567
Acknowledgments
Statistics supported in part by funds from David H. Koch provided through the Prostate Cancer Foundation, the Sidney Kimmel Center for Prostate and Urologic Cancers SPORE grant from the U.S. National Cancer Institute [P50-CA92629], and a Cancer Center Support Grant for the Cancer Therapy and Research Center at the University of Texas Health Science Center at San Antonio [P30-CA054174]. Grants to support the work of the ERSPC include: European Union Grants SOC 95 35109, SOC 96 201869 05F022, SOC 97 201329, SOC 98 32241, the 6th Framework Program of the EU: PMark:LSHC-CT-2004-503011; and The Dutch Cancer Society (KWF 94-869, 98-1657, 2002-277, 2006-3518); The Netherlands Organisation for Health Research and Development (ZonMW-002822820, 22000106, 50-50110-98-311); The Prostate Cancer Research Foundation of Rotterdam (SWOP); Beckman-Coulter-Hybritech Inc; Abbott Pharmaceuticals, Sweden; Af Jochnick’s foundation; Catarina and Sven Hagstroms family foundation; Gunvor and Ivan Svensson’s foundation; Johanniterorden, King Gustav V Jubilée Clinic Cancer Research Foundation; Sahlgrenska University Hospital; Schering Plough, Sweden, Swedish Cancer Society (Contract numbers 09 0107, 080315 and 083455); and Wallac Oy, Turkku, Finland. The Tyrol study is supported by the International Agency for Research on Cancer, Lyon and the Tyrolean Prostate Cancer Early Detection Group. The SABOR project is supported by the San Antonio Center of Biomarkers of Risk for Prostate Cancer CA086402. The ProtecT study is funded by the UK NIHR Health Technology Assessment Programme (projects 96/20/06, 96/20/99).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ankerst, D.P., Boeck, A., Freedland, S.J. et al. Evaluating the PCPT risk calculator in ten international biopsy cohorts: results from the Prostate Biopsy Collaborative Group. World J Urol 30, 181–187 (2012). https://doi.org/10.1007/s00345-011-0818-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-011-0818-5