Abstract
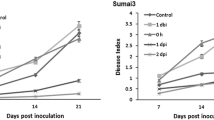
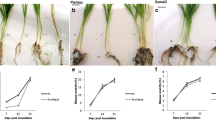
The Fusarium crown and root rot (FCRR) pathogen Fusarium culmorum (Fc) is a hemibiotrophic pathogen, with a short biotrophic phase preceding necrotropism. Methyl jasmonate (MeJA) has been identified as a vital growth regulator, and in the current study MeJA (200 μM) was applied at 2 days post Fc-inoculation (2 dpi) to compare wheat responses to F. culmorum challenge with responses induced by treatment with the plant defense signaling molecule during the necrotrophic stage. Our results show that a chemically induced protection significantly reduced necrotic symptoms in wheat cultivars over a 3 week period after inoculation. The activities of defense enzymes consisting of SOD, CAT, POX, PPO, LOX and PAL as well as total phenols and callose content, were enhanced earlier and to higher levels in tolerant cv Sumai3. In contrast to pathogen infection, there was not a general trend of an enhancement in cv Sumai3 following chemical treatment. In addition, MeJA significantly decreased the level of H2O2 contents and lipid peroxidation in all wheat cultivars studied. These results suggest that inducing JA dependent defense signaling after pathogen challenge may increase the resistance to FCRR by stimulating enzymatic activities and accumulation of phenolic compounds. This study apparently provides the first evidence of physiological and biochemical responses in wheat following MeJA treatment during the necrotrophic stage of infection. Based on the results, it can be concluded that there was a positive correlation between the wheat resistance levels and time of chemical treatment.





Similar content being viewed by others
References
Abeles FB, Biles CL (1991) Characterization of peroxidase in lignifying peach fruit endocarp. Plant Physiol 95:269–273
Aebi H (1974) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, New York, pp 673–677
Badawi GH, Yamauchi Y, Shimada E, Sasaki R, Kawano N, Tanaka K (2004) Enhanced tolerance to salt stress and water deficit by overexpressing superoxide dismutase in tobacco (Nicotiana tabacum) chloroplasts. Plant Sci 66:919–928
Bell E, Mullet JE (1993) Characterization of an Arabidopsis lipoxygenase gene responsive to methyl jasmonate and wounding. Plant Physiol 103:1133–1137
Bi JL, Felton GW (1995) Foliar oxidative stress and insect herbivory: primary compounds, secondary metabolites and reactive oxygen species as components of induced resistance. J Chem Ecol 21:1511–1530
Bradford MM (1976) A dye binding assay for protein. Anal Biochem 72:248–254
Broukanloui Madloo P, Behboudi K, Tohidfar M, Salehi Jouzani Gh, Ahmadzadeh M (2013) Response of some important Iranian wheat cultivars to Fusarium culmorum under genetic diversity of indigenous bio-control agent Fluorescent pseudomonas spp. Aust J Crop Sci 7(7):1003–1009
Constabel CP, Barbehenn R (2008) Defensive roles of polyphenol oxidase in plants. In: Schaller A (ed) Induced plant resistance to herbivory. Springer, New York, pp 253–270
Desmond OJ, Edgar CI, Manners JM, Maclean DJ, Schenk PM, Kazan K (2006) Methyl jasmonate induced gene expression in wheat delays symptom development by the crown rot pathogen Fusarium pseudograminearum. Physiol Mol Plant Pathol 67:171–179
Devi PUM, Reddy PS, Rani NU, Reddy K, Reddy MN, Reddanna P (2000) Lipoxygenase metabolites of α-linolenic acid in the development of resistance in Pigeon pea, Cajanus cajan (L.) mill sp, seedlings against Fusarium udum infection. Eur J Plant Pathol 106(9):857–865
Ding L, Xu H, Yi H, Yang L, Kong Z, Zhang L, Xue S, Jia H, Ma Z (2011) Resistance to hemi-biotrophic F. graminearum infection is associated with coordinated and ordered expression of diverse defense signaling pathways. PLoS ONE 6(4):e19008. doi:10.1371/journal.pone.0019008
Giannopolitis CN, Ries SK (1977) Superoxide dismutases I. Occurrence in higher plants. Plant Physiol 59(2):309–314
Grossman S, Zakut R (1979) Determination of the activity of lipoxygenase (lipoxidase). Methods Biochem 25:303–329
Hahlbrock K, Scheel D (1989) Physiology and molecular biology of phenylpropanoid metabolism. Ann Rev Plant Soil 40(1):347–369
Heath RL, Packer L (1986) Photoperoxidation in isolated chloroplasts. Arch Biochem Biophys 125:189–198
Helepciuc FE, Mitoi ME, Manole-Paunescu A, Aldea F, Brezeanu A, Cornea CP (2014) Induction of plant antioxidant system by interaction with beneficial and/or pathogenic microorganisms. Rom Biotech Lett 19:9366–9375
Jaiti F, Verdeil JL, Hadrami I (2009) Effect of jasmonic acid on the induction of polyphenoloxidase and peroxidase activities in relation to date palm resistance against Fusarium oxysporum f.sp. albedinis. Physiol Mol Plant Pathol 74:84–90
Jalalpour Z, Shabani L, Afghani L, Sharifi M, Amini SA (2014) Stimulatory effect of methyl jasmonate and squalestatin on phenolic metabolism through induction of LOX activity in cell suspension culture of yew. Turk J Biol 38:76–82
Kavitha R, Umesha S (2008) Regulation of defense-related enzymes associated with bacterial spot resistance in tomato. Phytoparasitica 36(2):144–159
Kohler A, Schwindling S, Conrath U (2002) Benzothiadiazole-induced priming for potentiated responses to pathogen infection, wounding, and infiltration of water into leaves require the NPR1/NIM1 gene in Arabidopsis. Plant Physiol 128(3):1046–1056
Lee DH, Lee CB (2000) Chilling stress-induced changes of antioxidant enzymes in the leaves of cucumber: in gel enzyme activity assays. Plant Sci 159(1):75–85
Li L, Steffens JC (2002) Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta 215:239–247
Li G, Yen Y (2008) Jasmonate and ethylene signaling pathway may mediate Fusarium head blight resistance in wheat. Crop Sci 48:1888–1896
Madadkhah E, Lotfi M, Nabipour A, Rahmanpour S, Banihashemi Z, Shoorooei M (2012) Enzymatic activities in roots of melon genotypes infected with Fusarium oxysporum f.sp. melonis race 1. Sci Hortic 135:171–176
Makandar R, Nalam VJ, Lee H, Trick HN, Dong Y, Shah J (2012) Salicylic acid regulates basal resistance to Fusarium head blight in wheat. Mol Plant Microbe Interact 25(3):431–439
Mhaske SD, Mahatma MK, Jha S, Singh P, Ahmad T (2013) Polyamine metabolism and lipoxygenase activity during Fusarium oxysporum f.sp. ricini-castor interaction. Physiol Mol Biol Plants 19:323–331
Mohammadi M, Kazemi H (2002) Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Sci 162:491–498
Motallebi P, Alkadri D, Pisi A, Nipoti P, Tonti S, Niknam V, Hashemi M, Prodi A (2015a) Pathogenicity and mycotoxin chemotypes of Iranian Fusarium culmorum isolates on durum wheat, and comparisons with Italian and Syrian isolates. Phytopathol Med 54(3):437–445
Motallebi P, Niknam V, Ebrahimzadeh H, Tahmasebi Enferadi S, Hashemi M (2015b) The effect of methyl jasmonate on enzyme activities in wheat genotypes infected by the crown and root rot pathogen Fusarium culmorum. Acta Physiol Plant 37:237
Motallebi P, Niknam V, Ebrahimzadeh H, Hashemi M, Pisi A, Prodi A, Tonti S, Nipoti P (2015c) Methyl jasmonate strengthens wheat plants against root and crown rot pathogen Fusarium culmorum infection. J Plant Growth Regul 34(3):624–636
Ochoa-Alejo N, Gómez-Peralta JE (1993) Activity of enzymes involved in capsaicin biosynthesis in callus tissue and fruits of chili pepper (Capsicum annuum). J Plant Physiol 141(2):147–152
Petti C, Reiber K, Ali SS, Berney M, Doohan FM (2012) Auxin as a player in the biocontrol of Fusarium head blight disease of barley and its potential as a disease control agent. BMC Plant Biol 12:224
Raymond J, Pakariyathan N, Azanza JL (1993) Purification and some properties of polyphenol oxidases from some flowers seed. Phytochem 34:927–931
Samia M, Khallal EL (2007) Induction and modulation of resistance in tomato plants against Fusarium wilt disease by bioagent fungi (Arbuscular mycorrhiza) and/or hormonal elicitors (jasmonic acid and salicylic acid) changes in the antioxidant enzymes, phenolic compounds and pathogen related-proteins. Aust J Basic Appl Sci 1(4):717–732
Scherm B, Balmas V, Spanu F, Pani G, Delogu G, Pasquali M, Migheli Q (2013) Fusarium culmorum: causal agent of foot and root rot and head blight on wheat. Mol Plant Pathol 14(4):323–341
Swain T, Hillis WE (1959) The phenolic constituents of Prumus domestica and quantitative analysis of phenolic constituents. J Sci Food Agric 10:63–68
Thomma B, Eggermont K, Broekaert WF, Cammue BPA (2000) Disease development of several fungi on Arabidopsis can be reduced by treatment with methyl jasmonate. Plant Physiol Biochem 38:421–427
Ton J, Mauch-Mani B (2004) β-amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose. Plant J 38(1):119–130
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151(1):59–66
Verhagen BWM, Glazebrook J, Zhu T, Chang HS, Van-Loon LC, Pieterse CMJ (2004) The transcriptome of rhizobacteria induced systemic resistance in Arabidopsis. Mol Plant Microbe Interact 17:895–908
Walters D, Cowley T, Mitchell A (2002) Methyl jasmonate alters polyamine metabolism and induces systemic protection against powdery mildew infection in barley seedlings. J Exp Bot 53:747–756
Wasternack C, Hause B (2002) Jasmonates and octadecanoids: signals in plant stress responses and development. Prog Nucl Acid Res Mol Biol 72:165–221
Wegener M (1992) Optimierung Von Saatgutpillierungen mit mikrobiellen antagonisten zur biologischen Bekampfung Von Fusarium culmorum in weizen. Diplomarbeit, Universitat Gottingen
Xu J, Duan X, Yang J, Beeching JR, Zhang P (2013) Enhanced reactive oxygen species scavenging by overproduction of superoxide dismutase and catalase delays postharvest physiological deterioration of cassava storage roots. Plant Physiol 161:1517–1528
Zeyen RJ, Carver TL, Lyngkjaer MF (2002) Epidermal cell papillae. In: Belanger RR, Bushnell WR, Dik AJ, Carver TLW (eds) The powdery mildews: a comprehensive treatise. APS Press, St Paul, pp 107–125
Acknowledgments
This work was supported by a Grant from University of Tehran. We thank the staff of School of Biology for their assistance in this experiment. We would like to thank Dr. Jutta Ludwig-Müller and the reviewing editor for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Motallebi, P., Niknam, V., Ebrahimzadeh, H. et al. Exogenous Methyl Jasmonate Treatment Induces Defense Response Against Fusarium culmorum in Wheat Seedlings. J Plant Growth Regul 36, 71–82 (2017). https://doi.org/10.1007/s00344-016-9620-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-016-9620-3