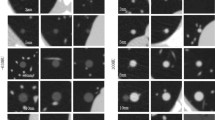
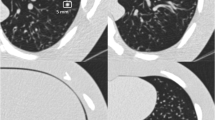
Abstract
Objectives
This study was conducted to evaluate the effect of dose reduction on the performance of a deep learning (DL)–based computer-aided diagnosis (CAD) system regarding pulmonary nodule detection in a virtual screening scenario.
Methods
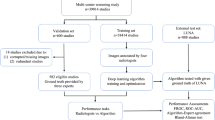
Sixty-eight anthropomorphic chest phantoms were equipped with 329 nodules (150 ground glass, 179 solid) with four sizes (5 mm, 8 mm, 10 mm, 12 mm) and scanned with nine tube voltage/current combinations. The examinations were analyzed by a commercially available DL-based CAD system. The results were compared by a comparison of proportions. Logistic regression was performed to evaluate the impact of tube voltage, tube current, nodule size, nodule density, and nodule location.
Results
The combination with the lowest effective dose (E) and unimpaired detection rate was 80 kV/50 mAs (sensitivity: 97.9%, mean false-positive rate (FPR): 1.9, mean CTDIvol: 1.2 ± 0.4 mGy, mean E: 0.66 mSv). Logistic regression revealed that tube voltage and current had the greatest impact on the detection rate, while nodule size and density had no significant influence.
Conclusions
The optimal tube voltage/current combination proposed in this study (80 kV/50 mAs) is comparable to the proposed combinations in similar studies, which mostly dealt with conventional CAD software. Modification of tube voltage and tube current has a significant impact on the performance of DL-based CAD software in pulmonary nodule detection regardless of their size and composition.
Key Points
• Modification of tube voltage and tube current has a significant impact on the performance of deep learning–based CAD software.
• Nodule size and composition have no significant impact on the software’s performance.
• The optimal tube voltage/current combination for the examined software is 80 kV/50 mAs.



Similar content being viewed by others
Change history
08 February 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00330-022-09350-w
Abbreviations
- AI:
-
Artificial intelligence
- CAD system:
-
Computer-aided diagnosis system
- CNR:
-
Contrast-to-noise ratio
- CT:
-
Computed tomography
- CTU:
-
Clinical trial unit
- DL:
-
Deep learning
- DLP:
-
Dose length product
- HFG:
-
Humanforschungsgesetz
- HFV:
-
Humanforschungsverordnung
- KV:
-
Kilovolt
- LCC:
-
Lung Cancer Center
- MIP:
-
Maximum intensity projection
- NLST:
-
National Lung Cancer Screening Trial
- OP:
-
Operation
- PACS:
-
Picture Archiving and Communication System
- PET-CT:
-
Positron-emission-tomography with computed tomography
- SNR:
-
Signal-to-noise ratio
- Sv:
-
Sievert
- T1 (a, b, c):
-
Tumor stage 1 (a, b, c)
References
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70:7–30
Aberle DR, Adams AM, Berg CD et al (2011) Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 365:395–409
Becker N, Motsch E, Gross ML et al (2015) Randomized study on early detection of lung cancer with MSCT in Germany: results of the first 3 years of follow-up after randomization. J Thorac Oncol 10:890–896
de Koning HJ, van der Aalst CM, de Jong PA et al (2020) Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 382:503–513
Paci E, Puliti D, Lopes Pegna A et al (2017) Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax 72:825–831
Brenner DJ (2004) Radiation risks potentially associated with low-dose CT screening of adult smokers for lung cancer. Radiology 231:440–445
Huber A, Landau J, Ebner L et al (2016) Performance of ultralow-dose CT with iterative reconstruction in lung cancer screening: limiting radiation exposure to the equivalent of conventional chest X-ray imaging. Eur Radiol 26:3643–3652
Messerli M, Kluckert T, Knitel M et al (2016) Computer-aided detection (CAD) of solid pulmonary nodules in chest x-ray equivalent ultralow dose chest CT - first in-vivo results at dose levels of 0.13mSv. Eur J Radiol 85:2217–2224
Neroladaki A, Botsikas D, Boudabbous S, Becker CD, Montet X (2013) Computed tomography of the chest with model-based iterative reconstruction using a radiation exposure similar to chest X-ray examination: preliminary observations. Eur Radiol 23:360–366
Kroft LJM, van der Velden L, Girón IH, Roelofs JJH, de Roos A, Geleijns J (2019) Added value of ultra–low-dose computed tomography, dose equivalent to chest X-ray radiography, for diagnosing chest pathology. J Thorac Imaging 34:179–186
Christe A, Charimo-Torrente J, Roychoudhury K, Vock P, Roos JE (2013) Accuracy of low-dose computed tomography (CT) for detecting and characterizing the most common CT-patterns of pulmonary disease. Eur J Radiol 82:e142-150
Kang S, Kim TH, Shin JM et al (2020) Optimization of a chest computed tomography protocol for detecting pure ground glass opacity nodules: a feasibility study with a computer-assisted detection system and a lung cancer screening phantom. PLoS One 15:e0232688
Solomon J, Mileto A, Nelson RC, Roy Choudhury K, Samei E (2016) Quantitative features of liver lesions, lung nodules, and renal stones at multi-detector row CT examinations: dependency on radiation dose and reconstruction algorithm. Radiology 279:185–194
Scholten ET, Horeweg N, de Koning HJ et al (2015) Computed tomographic characteristics of interval and post screen carcinomas in lung cancer screening. Eur Radiol 25:81–88
Torres EL, Fiorina E, Pennazio F et al (2015) Large scale validation of the M5L lung CAD on heterogeneous CT datasets. Med Phys 42:1477–1489
Christe A, Leidolt L, Huber A et al (2013) Lung cancer screening with CT: evaluation of radiologists and different computer assisted detection software (CAD) as first and second readers for lung nodule detection at different dose levels. Eur J Radiol 82:e873-878
Li L, Liu Z, Huang H, Lin M, Luo D (2019) Evaluating the performance of a deep learning-based computer-aided diagnosis (DL-CAD) system for detecting and characterizing lung nodules: comparison with the performance of double reading by radiologists. Thorac Cancer 10:183–192
Liang CH, Liu YC, Wu MT, Garcia-Castro F, Alberich-Bayarri A, Wu FZ (2020) Identifying pulmonary nodules or masses on chest radiography using deep learning: external validation and strategies to improve clinical practice. Clin Radiol 75:38–45
Setio AA, Ciompi F, Litjens G et al (2016) Pulmonary nodule detection in CT images: false positive reduction using multi-view convolutional networks. IEEE Trans Med Imaging 35:1160–1169
Tandon YK, Bartholmai BJ, Koo CW (2020) Putting artificial intelligence (AI) on the spot: machine learning evaluation of pulmonary nodules. J Thorac Dis 12:6954–6965
Zhao Y, de Bock GH, Vliegenthart R et al (2012) Performance of computer-aided detection of pulmonary nodules in low-dose CT: comparison with double reading by nodule volume. Eur Radiol 22:2076–2084
Wielpütz MO, Wroblewski J, Lederlin M et al (2015) Computer-aided detection of artificial pulmonary nodules using an ex vivo lung phantom: influence of exposure parameters and iterative reconstruction. Eur J Radiol 84:1005–1011
Blazis SP, Dickerscheid DBM, Linsen PVM, Martins Jarnalo CO (2021) Effect of CT reconstruction settings on the performance of a deep learning based lung nodule CAD system. Eur J Radiol 136:109526
Fu B, Wang G, Wu M et al (2020) Influence of CT effective dose and convolution kernel on the detection of pulmonary nodules in different artificial intelligence software systems: a phantom study. Eur J Radiol 126:108928
The, (2007) Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP 37:1–332
Shrimpton PC, Hillier MC, Lewis MA, Dunn M (2006) National survey of doses from CT in the UK: 2003. Br J Radiol 79:968–980
Ebner L, Roos JE, Christensen JD et al (2016) Maximum-intensity-projection and computer-aided-detection algorithms as stand-alone reader devices in lung cancer screening using different dose levels and reconstruction kernels. AJR Am J Roentgenol 207:282–288
Agresti A (2007) An introduction to categorical data analysis, 2nd edn. Wiley-Interscience, Hoboken, NJ
Ebner L, Bütikofer Y, Ott D et al (2015) Lung nodule detection by microdose CT versus chest radiography (standard and dual-energy subtracted). AJR Am J Roentgenol 204:727–735
Christe A, Szucs-Farkas Z, Huber A et al (2013) Optimal dose levels in screening chest CT for unimpaired detection and volumetry of lung nodules, with and without computer assisted detection at minimal patient radiation. PLoS One 8:e82919
Hua KL, Hsu CH, Hidayati SC, Cheng WH, Chen YJ (2015) Computer-aided classification of lung nodules on computed tomography images via deep learning technique. Onco Targets Ther 8:2015–2022
Christe A, Torrente JC, Lin M et al (2011) CT screening and follow-up of lung nodules: effects of tube current-time setting and nodule size and density on detectability and of tube current-time setting on apparent size. AJR Am J Roentgenol 197:623–630
Acknowledgements
Dr. Peters reports grants from clinical trial unit (CTU) Bern and European School of Radiology (ESOR) outside the submitted work.
Dr. Heverhagen reports grants from Bayer Healthcare AG, grants from Guerbet AG, grants from Siemens Healthineers, and grants from Bracco Imaging Spa outside the submitted work.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Peters.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise (Dr. Christe).
Informed consent
Written informed consent was waived by the Institutional Review Board (phantom study).
Ethical approval
Institutional Review Board approval was not required for this phantom study.
Study subjects or cohorts overlap
Some study subjects or cohorts (chest phantoms) have been reported in a previous publication:
Huber A, Landau J, Ebner L et al. (2016) Performance of ultralow-dose CT with iterative reconstruction in lung cancer screening: limiting radiation exposure to the equivalent of conventional chest X-ray imaging. Eur Radiol 26:3643–3652.
Methodology
• Phantom study
• Diagnostic or prognostic study
• Performed at one institution
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: the figures 2 & 3 have been replaced by their correct versions.
The original online version of this article was revised: the figures 1, 2 & 3 have been replaced by their correct versions.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peters, A.A., Huber, A.T., Obmann, V.C. et al. Diagnostic validation of a deep learning nodule detection algorithm in low-dose chest CT: determination of optimized dose thresholds in a virtual screening scenario. Eur Radiol 32, 4324–4332 (2022). https://doi.org/10.1007/s00330-021-08511-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08511-7