Abstract
Objective
The aim of this study was (1) to investigate the application of texture analysis of choline PET/CT images in prostate cancer (PCa) patients and (2) to propose a machine-learning radiomics model able to select PET features predictive of disease progression in PCa patients with a same high-risk class at restaging.
Material and methods
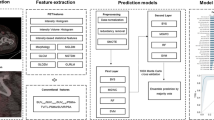
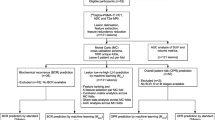
Ninety-four high-risk PCa patients who underwent restaging Cho-PET/CT were analyzed. Follow-up data were recorded for a minimum of 13 months after the PET/CT scan. PET images were imported in LIFEx toolbox to extract 51 features from each lesion. A statistical system based on correlation matrix and point-biserial-correlation coefficient has been implemented for features reduction and selection, while Discriminant analysis (DA) was used as a method for features classification in a whole sample and sub-groups for primary tumor or local relapse (T), nodal disease (N), and metastatic disease (M).
Results
In the whole group, 2 feature (HISTO_Entropy_log10; HISTO_Energy_Uniformity) results were able to discriminate the occurrence of disease progression at follow-up, obtaining the best performance in DA classification (sensitivity 47.1%, specificity 76.5%, positive predictive value (PPV) 46.7%, and accuracy 67.6%). In the sub-group analysis, the best performance in DA classification for T was obtained by selecting 3 features (SUVmin; SHAPE_Sphericity; GLCM_Correlation) with a sensitivity of 91.6%, specificity 84.1%, PPV 79.1%, and accuracy 87%; for N by selecting 2 features (HISTO = _Energy Uniformity; GLZLM_SZLGE) with a sensitivity of 68.1%, specificity 91.4%, PPV 83%, and accuracy 82.6%; and for M by selecting 2 features (HISTO_Entropy_log10 - HISTO_Entropy_log2) with a sensitivity 64.4%, specificity 74.6%, PPV 40.6%, and accuracy 72.5%.
Conclusion
This machine learning model demonstrated to be feasible and useful to select Cho-PET features for T, N, and M with valuable association with high-risk PCa patients’ outcomes.
Key Points
• Artificial intelligence applications are feasible and useful to select Cho-PET features.
• Our model demonstrated the presence of specific features for T, N, and M with valuable association with high-risk PCa patients’ outcomes.
• Further prospective studies are necessary to confirm our results and to develop the application of artificial intelligence in PET imaging of PCa.




Similar content being viewed by others
Abbreviations
- ADT:
-
Anti-androgen enzalutamide or abiraterone
- AUROC:
-
Area under the receiver operating characteristics
- BS:
-
Bone scan
- ceCT:
-
Contrast-enhanced computed tomography
- Cho-PET:
-
18F-choline PET/CT
- CHT:
-
Docetaxel or cabazitaxel or estramustine
- CI:
-
Confidence interval
- DA:
-
Discriminant analysis
- FU:
-
Follow-up
- GLZLM:
-
Grey-level zone length matrix
- HISTO:
-
Histogram
- HT:
-
Hormone therapy
- M:
-
Bone metastasis
- MR:
-
Magnetic resonance
- N:
-
Lymph-nodal disease
- PCa:
-
Prostate cancer
- PET:
-
Positron emission tomography
- PPV:
-
Positive predictive value
- RT:
-
Radiotherapy
- SUV:
-
Standardized uptake value
- SZLGE:
-
Short-zone low grey-level emphasis
- T:
-
Primary or local relapse
References
Zamboglou C, Carles M, Fechter T et al (2019) Radiomic features from PSMA PET for non-invasive intraprostatic tumor discrimination and characterization in patients with intermediate- and high-risk prostate cancer - a comparison study with histology reference. Theranostics 9:2595–2605. https://doi.org/10.7150/thno.32376
Li P, Wang X, Xu C et al (2020) 18F-FDG PET/CT radiomic predictors of pathologic complete response (pCR) to neoadjuvant chemotherapy in breast cancer patients. Eur J Nucl Med Mol Imaging 47:1116–1126. https://doi.org/10.1007/s00259-020-04684-3
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577. https://doi.org/10.1148/radiol.2015151169
Laufer M, Pound CR, Carducci MA, Eisenberger MA (2000) Management of patients with rising prostate-specific antigen after radical prostatectomy. Urology 55:309–315
Moul JW (2000) Prostate specific antigen only progression of prostate cancer. J Urol 163:1632–1642
Shikanov S, Kocherginsky M, Shalhav AL, Eggener SE (2012) Cause-specific mortality following radical prostatectomy. Prostate Cancer Prostatic Dis 15:106–110. https://doi.org/10.1038/pcan.2011.55
Zumsteg ZS, Spratt DE, Romesser PB et al (2015) Anatomical patterns of recurrence following biochemical relapse in the dose escalation era of external beam radiotherapy for prostate cancer. J Urol 194:1624–1630. https://doi.org/10.1016/j.juro.2015.06.100
Chowdhury R, Ganeshan B, Irshad S et al (2014) The use of molecular imaging combined with genomic techniques to understand the heterogeneity in cancer metastasis. Br J Radiol 87:20140065. https://doi.org/10.1259/bjr.20140065
Hatt M, Tixier F, Visvikis D, Cheze Le Rest C (2017) Radiomics in PET/CT: more than meets the eye? J Nucl Med 58:365–366. https://doi.org/10.2967/jnumed.116.184655
Incerti E, Fodor A, Mapelli P et al (2015) Radiation treatment of lymph node recurrence from prostate cancer: is 11C-choline PET/CT predictive of survival outcomes? J Nucl Med 56. https://doi.org/10.2967/jnumed.115.163741
ALONGI P, Stefano A, Comelli A et al (2020) New artificial intelligence model for 18F-choline PET/CT in evaluation of high-risk prostate cancer outcome: texture analysis and radiomics features classification for prediction of disease progression. J Nucl Med 61:1303–1303
Alongi P, Laudicella R, Stefano A et al (2020) Choline PET/CT features to predict survival outcome in high risk prostate cancer restaging: a preliminary machine-learning radiomics study. Q J Nucl Med Mol Imaging. https://doi.org/10.23736/S1824-4785.20.03227-6
Zhang Y, Oikonomou A, Wong A, Haider MA, Khalvati F (2017) Radiomics-based prognosis analysis for non-small cell lung cancer. Sci Rep 7:46349. https://doi.org/10.1038/srep46349
Belli ML, Mori M, Broggi S et al (2018) Quantifying the robustness of [18F]FDG-PET/CT radiomic features with respect to tumor delineation in head and neck and pancreatic cancer patients. Phys Med 49:105–111. https://doi.org/10.1016/J.EJMP.2018.05.013
Bogowicz M, Vuong D, Huellner MW et al (2019) CT radiomics and PET radiomics: ready for clinical implementation? Q J Nucl Med Mol Imaging 63. https://doi.org/10.23736/S1824-4785.19.03192-3
D’Amico AV, Whittington R, Malkowicz SB et al (1998) Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280:969–974
Cornford P, Bellmunt J, Bolla M et al (2017) EAU-ESTRO-SIOG Guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol 71:630–642. https://doi.org/10.1016/j.eururo.2016.08.002
Comelli A, Stefano A, Russo G et al (2018) A smart and operator independent system to delineate tumours in positron emission tomography scans. Comput Biol Med. https://doi.org/10.1016/J.COMPBIOMED.2018.09.002
Nioche C, Orlhac F, Boughdad S et al (2018) LIFEx: A freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res 78:4786–4789. https://doi.org/10.1158/0008-5472.CAN-18-0125
Comelli A, Stefano A, Coronnello C, et al (2020) Radiomics: a new biomedical workflow to create a predictive model. In: Communications in Computer and Information Science. Springer, pp 280–293
Comelli A, Stefano A, Bignardi S et al (2019) Active contour algorithm with discriminant analysis for delineating tumors in positron emission tomography. Artif Intell Med 94:67–78. https://doi.org/10.1016/j.artmed.2019.01.002
Comelli A, Bignardi S, Stefano A et al (2020) Development of a new fully three-dimensional methodology for tumours delineation in functional images. Comput Biol Med 120:103701. https://doi.org/10.1016/j.compbiomed.2020.103701
Comelli A, Stefano A, Russo G et al (2019) K-nearest neighbor driving active contours to delineate biological tumor volumes. Eng Appl Artif Intel 81:133–144. https://doi.org/10.1016/j.engappai.2019.02.005
Comelli A, Stefano A, Bignardi S, et al (2020) Tissue classification to support local active delineation of brain tumors. In: Communications in Computer and Information Science. Springer, pp 3–14
Schatten H (2018) Brief overview of prostate cancer statistics, grading, diagnosis and treatment strategies. Springer, Cham, pp 1–14
Klotz L (2013) Active surveillance for prostate cancer: overview and update. Curr Treat Options Oncol 14:97–108. https://doi.org/10.1007/s11864-012-0221-5
Boustani AM, Pucar D, Saperstein L (2018) Molecular imaging of prostate cancer. Br J Radiol 20170736. https://doi.org/10.1259/bjr.20170736
Laudicella R, Albano D, Alongi P et al (2019) 18F-Facbc in prostate cancer: a systematic review and meta-analysis. Cancers (Basel) 11:1348. https://doi.org/10.3390/cancers11091348
Ghafoor S, Burger IA, Vargas AH (2019) Multimodality imaging of prostate cancer. J Nucl Med 60:1350–1358. https://doi.org/10.2967/JNUMED.119.228320
De Bari B, Mazzola R, Aiello D et al (2018) Could 68-Ga PSMA PET/CT become a new tool in the decision-making strategy of prostate cancer patients with biochemical recurrence of PSA after radical prostatectomy? A preliminary, monocentric series. Radiol Med 123. https://doi.org/10.1007/s11547-018-0890-7
Fiorentino A, Laudicella R, Ciurlia E et al (2019) Positron emission tomography with computed tomography imaging (PET/CT) for the radiotherapy planning definition of the biological target volume: PART 2. Crit Rev Oncol Hematol 139:117–124. https://doi.org/10.1016/J.CRITREVONC.2019.03.008
Mapelli P, Incerti E, Ceci F et al (2016) 11C- or 18F-choline PET/CT for imaging evaluation of biochemical recurrence of prostate cancer. J Nucl Med 57:43S–48S. https://doi.org/10.2967/jnumed.115.169755
Baratto L, Duan H, Laudicella R et al (2020) Physiological 68 Ga-RM2 uptake in patients with biochemically recurrent prostate cancer: an atlas of semi-quantitative measurements. EJNMMI 47:115–122 https://link.springer.com/article/10.1007/s00259-019-04503-4
Dercle L, Ammari S, Bateson M et al (2017) Limits of radiomic-based entropy as a surrogate of tumor heterogeneity: ROI-area, acquisition protocol and tissue site exert substantial influence. Sci Rep 7. https://doi.org/10.1038/S41598-017-08310-5
Lubner MG, Stabo N, Abel EJ et al (2016) CT textural analysis of large primary renal cell carcinomas: pretreatment tumor heterogeneity correlates with histologic findings and clinical outcomes. AJR Am J Roentgenol 207:96–105. https://doi.org/10.2214/AJR.15.15451
Acar E, Leblebici A, Ellidokuz BE et al (2019) Machine learning for differentiating metastatic and completely responded sclerotic bone lesion in prostate cancer: a retrospective radiomics study. Br J Radiol 92:20190286. https://doi.org/10.1259/bjr.20190286
Acknowledgments
Thanks to the President of Fondazione Istituto G. Giglio Dr. Salvatore Albano for providing to the Hospital, included Nuclear Medicine Unit, the motivation and the support needed for high quality in clinical and scientific nuclear medicine procedures.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Pierpaolo Alongi, Head of Nuclear Medicine Unit at Fondazione Istituto Giglio of Cefalù, Italy.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Two authors have significant statistical expertise (Alessandro Stefano, Albert Comelli).
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported as SNMMI 2020 Congress presentation in “Alongi P, Stefano A, Comelli A, et al (2020) New Artificial intelligence model for 18F-Choline PET/CT in evaluation of high-risk prostate cancer outcome: texture analysis and radiomics features classification for prediction of disease progression. J Nucl Med 61:1303–1303.”
Methodology
• retrospective
• diagnostic or prognostic study/observational/experimental
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alongi, P., Stefano, A., Comelli, A. et al. Radiomics analysis of 18F-Choline PET/CT in the prediction of disease outcome in high-risk prostate cancer: an explorative study on machine learning feature classification in 94 patients. Eur Radiol 31, 4595–4605 (2021). https://doi.org/10.1007/s00330-020-07617-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07617-8