Abstract
Purpose
To evaluate therapeutic efficacy and complication of percutaneous thermal ablation of subcapsular hepatocellular carcinomas (HCCs), and how these may be influenced by the degree of tumor to liver surface contact and tumor protrusion from liver surface.
Materials and methods
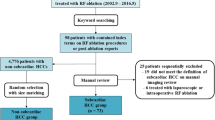
Our retrospective study was approved by the Institutional Review Board. Between January 2006 and December 2013, 290 patients (82 women, 208 men; mean age, 64.5 years; range, 33–89 years) with 474 subcapsular (within 1 cm to the liver surface) HCCs (mean size, 23.7 mm; range, 6–71 mm) underwent percutaneous thermal ablation. The HCCs were divided into surface contact group (n = 243) and non-surface contact group (n = 231). The former was further subdivided into exophytic and non-exophytic HCCs. Technical success, primary technique efficacy, local tumor progression (LTP), and secondary technique efficacy rates were analyzed and compared by the chi-square test or Fisher exact test. Prognostic factors for LTP and secondary technique efficacy were assessed using the Cox regression model. Major complications were also assessed.
Results
With median follow-up of 15 months (range, 1–87 months), technical success and primary technique efficacy were 98.7% and 95.7% % in the non-surface contact group; 96.4% and 94.0% in the non-exophytic group; and 100% and 94.7% in the exophytic group (p > 0.05). Tumor size > 3 cm was a significant predictor for LTP, but not for secondary efficacy. Overall major complication rate was 3.8% (24/624) and was not different among the three groups.
Conclusion
Subcapsular HCCs can be effectively treated with thermal ablation techniques. Degree of tumor-surface contact including moderate protrusion does not appear to limit feasibility or procedure effectiveness.
Key Points
• Subcapsular HCCs can be effectively treated with thermal therapy when proper image-guided technique and assistive techniques are applied.
• Degree of tumor surface contact including moderate protrusion does not appear to limit feasibility or procedure effectiveness.
• Major complications after percutaneous thermal ablation of subcapsular HCCs such as tumor seeding can be minimized by avoiding breach of the tumor capsule exposed to the peritoneal surface and use of tract ablation.


Similar content being viewed by others
Abbreviations
- AFP:
-
Alpha-fetoprotein
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- HCC:
-
Hepatocellular carcinoma
- HR:
-
Hazard ratio
- LTP:
-
Local tumor progression
- MRI:
-
Magnetic resonance imaging
- MWA:
-
Microwave ablation
- RFA:
-
Radiofrequency ablation
- TACE:
-
Transcatheter arterial chemoembolization
- US:
-
Ultrasonography
References
Bruix J, Sherman M, American Association for the Study of Liver Diseases (2011) Management of hepatocellular carcinoma: an update. Hepatology 53:1020–1022
European Association For The Study Of The Liver, European Organisation For Research and Treatment Of Cancer (2012) EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56:908–943. https://doi.org/10.1016/j.jhep.2011.12.001
Forner A, Llovet JM, Bruix J (2012) Hepatocellular carcinoma. Lancet 379:1245–1255
Seror O, N’Kontchou G, Ibraheem M et al (2008) Large (>or = 5.0-cm) HCCs: multipolar RF ablation with three internally cooled bipolar electrodes--initial experience in 26 patients. Radiology 248:288–296
Thomasset SC, Dennison AR, Garcea G (2015) Ablation for recurrent hepatocellular carcinoma: a systematic review of clinical efficacy and prognostic factors. World J Surg 39:1150–1160
Komorizono Y, Oketani M, Sako K et al (2003) Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer 97:1253–1262
Kim YJ, Raman SS, Yu NC, Busuttil RW, Tong M, Lu DS (2008) Radiofrequency ablation of hepatocellular carcinoma: can subcapsular tumors be safely ablated? AJR Am J Roentgenol 190:1029–1034
Kang TW, Lim HK, Lee MW et al (2016) Long-term therapeutic outcomes of radiofrequency ablation for subcapsular versus nonsubcapsular hepatocellular carcinoma: a propensity score matched study. Radiology 280:300–312
Llovet JM, Vilana R, Bru C et al (2001) Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology 33:1124–1129
Teratani T, Yoshida H, Shiina S et al (2006) Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology 43:1101–1108
Francica G, Meloni MF, de Sio I et al (2016) Radiofrequency and microwave ablation of subcapsular hepatocellular carcinoma accessed by direct puncture: safety and efficacy. Eur J Radiol 85:739–743
Filippousis P, Sotiropoulou E, Manataki A, Konstantinopoulos O, Thanos L (2011) Radiofrequency ablation of subcapsular hepatocellular carcinoma: single center experience. Eur J Radiol 77:299–304
Sartori S, Tombesi P, Macario F et al (2008) Subcapsular liver tumors treated with percutaneous radiofrequency ablation: a prospective comparison with nonsubcapsular liver tumors for safety and effectiveness. Radiology 248:670–679
Mitchell DG, Bruix J, Sherman M, Sirlin CB (2015) LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology 61:1056–1065
McWilliams JP, Plotnik AN, Sako EY et al (2014) Safety of hydroinfusion in percutaneous thermal ablation of hepatic malignancies. J Vasc Interv Radiol 25:1118–1124
Sacks D, McClenny TE, Cardella JF, Lewis CA (2003) Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 14:S199–S202
Ahmed M, Technology Assessment Committee of the Society of Interventional Radiology (2014) Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update: supplement to the consensus document. J Vasc Interv Radiol 25:1706–1708
Hori T, Nagata K, Hasuike S et al (2003) Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J Gastroenterol 38:977–981
Jaskolka JD, Asch MR, Kachura JR et al (2005) Needle tract seeding after radiofrequency ablation of hepatic tumors. J Vasc Interv Radiol 16:485–491
Imamura J, Tateishi R, Shiina S et al (2008) Neoplastic seeding after radiofrequency ablation for hepatocellular carcinoma. Am J Gastroenterol 103:3057–3062
Yu J, Liang P, Yu XL, Cheng ZG, Han ZY, Dong BW (2012) Needle track seeding after percutaneous microwave ablation of malignant liver tumors under ultrasound guidance: analysis of 14-year experience with 1462 patients at a single center. Eur J Radiol 81:2495–2499
Rhim H, Yoon KH, Lee JM et al (2003) Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics 23:123–134 discussion 134-126
Head HW, Dodd GD 3rd, Dalrymple NC et al (2007) Percutaneous radiofrequency ablation of hepatic tumors against the diaphragm: frequency of diaphragmatic injury. Radiology 243:877–884
Song I, Rhim H, Lim HK, Kim YS, Choi D (2009) Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol 19:2630–2640
Wang CC, Kao JH (2015) Artificial ascites is feasible and effective for difficult-to-ablate hepatocellular carcinoma. Hepatol Int 9:514–519
Yang W, Yan K, Wu GX et al (2015) Radiofrequency ablation of hepatocellular carcinoma in difficult locations: strategies and long-term outcomes. World J Gastroenterol 21:1554–1566
Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN (2003) Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 226:441–451
Patel PA, Ingram L, Wilson ID, Breen DJ (2013) No-touch wedge ablation technique of microwave ablation for the treatment of subcapsular tumors in the liver. J Vasc Interv Radiol 24:1257–1262
Rossi S, Ravetta V, Rosa L et al (2011) Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology 53:136–147
Feng K, Yan J, Li X et al (2012) A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 57:794–802
Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST (2004) Radiofrequency ablation for subcapsular hepatocellular carcinoma. Ann Surg Oncol 11:281–289
Cillo U, Vitale A, Dupuis D et al (2013) Laparoscopic ablation of hepatocellular carcinoma in cirrhotic patients unsuitable for liver resection or percutaneous treatment: a cohort study. PLoS One 8:e57249
Santambrogio R, Bruno S, Kluger MD et al (2016) Laparoscopic ablation therapies or hepatic resection in cirrhotic patients with small hepatocellular carcinoma. Dig Liver Dis 48:189–196
Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L (2005) Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg 242:158–171
de la Serna S, Vilana R, Sanchez-Cabus S et al (2015) Results of laparoscopic radiofrequency ablation for HCC. Could the location of the tumour influence a complete response to treatment? A single European centre experience. HPB (Oxford) 17:387–393
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is David S. Lu, MD.
Conflict of interest
The authors of this manuscript declare relationships with the following companies:
David Lu, MD., has received funding for education and research, or consulting from Rita Medical, Boston Scientific, Covidien, and NeuWave Medical.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in:
1. Lee MW, Raman SS, Asvadi NH, Siripongsakun S, Hicks RM, Chen J, Worakitsitisatorn A, McWilliams J, Tong MJ, Finn RS, Agopian VG, Busuttil RW, Lu DSK. Radiofrequency ablation of hepatocellular carcinoma as bridge therapy to liver transplantation: A 10-year intention-to-treat analysis. Hepatology. 2017 Jun;65(6):1979-1990. doi: 10.1002/hep.29098. Epub 2017 Apr 28. PubMed PMID: 28170115.
Above study included patients who were listed for liver transplantation only and all tumor locations within the liver, while the current manuscript addresses only subcapsular HCC subjects, with majority not liver transplant candidates.
2. Thamtorawat S, Hicks RM, Yu J, Siripongsakun S, Lin WC, Raman SS, McWilliams
JP, Douek M, Bahrami S, Lu DS. Preliminary Outcome of Microwave Ablation of Hepatocellular Carcinoma: Breaking the 3-cm Barrier? J Vasc Interv Radiol. 2016 May;27(5):623-30. doi: 10.1016/j.jvir.2016.01.011. Epub 2016 Mar 21. PubMed PMID: 27013403.
Above article included only microwave ablation patients but all liver treatment sites, while the current manuscript addresses only subcapsular locations and includes both microwave and radiofrequency ablation subjects.
Methodology
• Retrospective
• Observational
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Table 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Worakitsitisatorn, A., Lu, D.S., Lee, M.W. et al. Percutaneous thermal ablation of subcapsular hepatocellular carcinomas: influence of tumor-surface contact and protrusion on therapeutic efficacy and safety. Eur Radiol 30, 1813–1821 (2020). https://doi.org/10.1007/s00330-019-06497-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06497-x