Abstract
Aim
To describe the magnetic resonance imaging (MRI) features of HIV-associated obliterative portopathy (HIV-OP) and determine the most indicative appearance of this condition on MRI by using a retrospective case-control study.
Methods
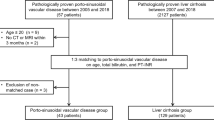
MRI examinations of 24 patients with HIV-OP (16 men, 8 women; mean age = 48 ± 6.6 [SD] years; age range, 35–71 years) were analyzed by two blinded observers and compared with those obtained in 18 HIV-infected patients with hepatic cirrhosis (14 men, 4 women; mean age = 51 ± 3.4 [SD] years; age range, 35–60 years). Images were qualitatively and quantitatively analyzed with respect to imaging presentation. Comparisons were performed using uni- and multivariate analyses.
Results
Regular liver contours had the highest accuracy for the diagnosis of HIV-OP (83%, 35 of 42; 95% confidence interval [CI], 69–93%) and was the most discriminating independent variable for the diagnosis of HIV-OP (odds ratio, 51; 95%CI, 4.96–1272%) (p < 0.0001). At multivariate analysis, the width of segment 4 in millimeters (OR = 1.23 [95%CI, 1.05–1.44%]; p = 0.011) and the presence of regular liver contours (OR = 7.69 [95%CI, 1.48–39.92%]; p = 0.015) were the variables independently associated with the diagnosis of HIV-OP.
Conclusions
Regular liver contours are the most discriminating independent variable for the diagnosis of HIV-OP but have limited accuracy. Familiarity with this finding may help differentiate HIV-OP from cirrhosis in HIV-infected patients.
Key Points
• Regular liver contour is the most discriminating independent variable for the diagnosis of HIV-OP (odds ratio = 51) with 83% accuracy.
• At multivariate analysis, the width of segment 4 in millimeters and the presence of regular liver contours are the variables independently associated with the diagnosis of HIV-OP.
• MRI helps diagnose HIV-OP in the presence of several categorical findings, which are more frequently observed in HIV-OP patients than in HIV patients with cirrhosis.






Similar content being viewed by others
Abbreviations
- 3D VIBE:
-
Three-dimensional volumetric interpolated breath-hold gradient-echo
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- CL/RL:
-
Caudate-to-right lobe ratio
- CT:
-
Computed tomography
- FNH:
-
Focal nodular hyperplasia
- HASTE:
-
T2-weighted half-Fourier acquisition single-shot turbo spin-echo
- HIV:
-
Human immunodeficiency virus
- MRI:
-
Magnetic resonance imaging
- OP:
-
Obliterative portopathy
- PACS:
-
Picture archiving and communication system
- ROC:
-
Receiving operative curve
- SD:
-
Standard deviation
- SI:
-
Splenic index
- TSE :
-
Turbo spin-echo
References
Khanna R, Sarin SK (2014) Non-cirrhotic portal hypertension - diagnosis and management. J Hepatol 60:421–441
Rajesh S, Mukund A, Sureka B, Bansal K, Ronot M, Arora A (2018) Non-cirrhotic portal hypertension: an imaging review. Abdom Radiol 43:1991–2010
Krasinskas AM, Eghtesad B, Kamath PS, Demetris AJ, Abraham SC (2005) Liver transplantation for severe intrahepatic noncirrhotic portal hypertension. Liver Transpl 11:627–634
Isabel Fiel M, Thung SN, Hytiroglou P, Emre S, Schiano TD (2007) Liver failure and need for liver transplantation in patients with advanced hepatoportal sclerosis. Am J Surg Pathol 31:607–614
Nakanuma Y, Tsuneyama K, Ohbu M, Katayanagi K (2001) Pathology and pathogenesis of idiopathic portal hypertension with an emphasis on the liver. Pathol Res Pract 197:65–76
Hillaire S, Bonte E, Denninger MH et al (2002) Idiopathic non-cirrhotic intrahepatic portal hypertension in the west: a re-evaluation in 28 patients. Gut 51:275–280
Vispo E, Morello J, Rodriguez-Novoa S, Soriano V (2011) Noncirrhotic portal hypertension in HIV infection. Curr Opin Infect Dis 24:12–18
Krishnan P, Fiel MI, Rosenkrantz AB et al (2012) Hepatoportal sclerosis: CT and MRI appearance with histopathologic correlation. AJR Am J Roentgenol 198:370–376
Wanless IR (1990) Micronodular transformation (nodular regenerative hyperplasia) of the liver: a report of 64 cases among 2,500 autopsies and a new classification of benign hepatocellular nodules. Hepatology 11:787797
Mallet V, Blanchard P, Verkarre V et al (2007) Nodular regenerative hyperplasia is a new cause of chronic liver disease in HIV-infected patients. AIDS 21:187–192
Mallet VO, Varthaman A, Lasne D et al (2009) Acquired protein S deficiency leads to obliterative portal venopathy and to compensatory nodular regenerative hyperplasia in HIV-infected patients. AIDS 23:1511–1518
Solis-Herruzo JA, Vidal JV, Colina F, Santalla F, Castellano G (1986) Nodular regenerative hyperplasia of the liver associated with the toxic oil syndrome: report of five cases. Hepatology 6:687–693
Seiderer J, Zech CJ, Reinisch W et al (2005) A multicenter assessment of liver toxicity by MRI and biopsy in IBD patients on 6-thioguanine. J Hepatol 43:303–309
Bajaj JS, Bhattacharjee J, Sarin SK (2001) Coagulation profile and platelet function in patients with extrahepatic portal vein obstruction and non-cirrhotic portal fibrosis. J Gastroenterol Hepatol 16:641–646
Maida I, Núñez M, Ríos MJ et al (2006) Severe liver disease associated with prolonged exposure to antiretroviral drugs. J Acquir Immune Defic Syndr 42:177–182
Dinh MH, Stosor V, Rao SM, Miller FH, Green RM (2009) Cryptogenic liver disease in HIV-seropositive men. HIV Med 10:447–453
Mallet VO, Vallet-Pichard A, Pol S (2009) Human immunodeficiency virus-associated obliterative portopathy underlies unexplained aminotransferase elevations under antiretrovirals. Hepatology 50:660
Mallet VO, Sultanik PS, Vallet-Pichard A, Pol S (2010) HIV-associated obliterative portopathy (HIV-OP) underlies cryptogenic liver disease in HIV-seropositive patients. HIV Med 11:540–541
Schouten JN, Van der Ende ME, Koëter T et al (2012) Risk factors and outcome of HIV-associated idiopathic noncirrhotic portal hypertension. Aliment Pharmacol Ther 36:875–885
Saifee S, Joelson D, Braude J et al (2008) Noncirrhotic portal hypertension in patients with human immunodeficiency virus-1 infection. Clin Gastroenterol Hepatol 6:1167–1169
Wanless IR, Peterson P, Das A, Boitnott JK, Moore GW, Bernier V (1990) Hepatic vascular disease and portal hypertension in polycythemia vera and agnogenic myeloid metaplasia: a clinicopathological study of 145 patients examined at autopsy. Hepatology 12:1166–1174
Wanless IR, Godwin TA, Allen F, Feder A (1980) Nodular regenerative hyperplasia of the liver in hematologic disorders: a possible response to obliterative portal venopathy. A morphometric study of nine cases with an hypothesis on the pathogenesis. Medicine (Baltimore) 59:36779
Glatard AS, Hillaire S, d’Assignies G et al (2012) Obliterative portal venopathy: findings at CT imaging. Radiology 263:741–750
Laharie D, Vergniol J, Bioulac-Sage P et al (2010) Usefulness of noninvasive tests in nodular regenerative hyperplasia of the liver. Eur J Gastroenterol Hepatol 22:487–493
Zech CJ, Seiderer J, Reinisch W et al (2007) Thioguanin-induced nodular regenerative hyperplasia of the liver-ROC analysis of different MR techniques. Eur Radiol 17:1898–1905
Arora A, Sarin SK (2015) Multimodality imaging of obliterative portal venopathy: what every radiologist should know. Br J Radiol 88:20140653
Mikkelsen WP, Edmondson HA, Peters RL, Redeker AG, Reynolds TB (1965) Extra- and intrahepatic portal hypertension without cirrhosis (hepatoportal sclerosis). Ann Surg 162:602–620
Kirchgesner T, Perlepe V, Michoux N, Larbi A, Vande Berg B (2018) Fat suppression at three-dimensional T1-weighted MR imaging of the hands: Dixon method versus CHESS technique. Diagn Interv Imaging 99:23–28
Soyer P, Sirol M, Dohan A et al (2012) Hepatic height on coronal computed tomography images predicts total liver volume in European adults without liver disease. Dig Dis Sci 57:1692–1697
Lafortune M, Matricardi L, Denys A, Favret M, Déry R, Pomier-Layrargues G (1998) Segment 4 (the quadrate lobe): a barometer of cirrhotic liver disease at US. Radiology 206:157–160
Prassopoulos P, Daskalogiannaki M, Raissaki M, Hatjidakis A, Gourtsoyiannis N (1991) Determination of normal splenic volume on computed tomography in relation to age, gender and body habitus. Eur Radiol 7:246–248
Awaya H, Mitchell DG, Kamishima T, Holland G, Ito K, Matsumoto T (2002) Cirrhosis: modified caudate-right lobe ratio. Radiology 224:769–774
Weinreb J, Kumari S, Phillips G, Pochaczevsky R (1982) Portal vein measurements by real-time sonography. AJR Am J Roentgenol 139:497–499
Moriyasu F, Ban N, Nishida O et al (1986) Clinical application of an ultrasonic duplex system in the quantitative measurement of portal blood flow. J Clin Ultrasound 14:579–588
Brancatelli G, Federle MP, Ambrosini R et al (2007) Cirrhosis: CT and MR imaging evaluation. Eur J Radiol 61:57–69
Tonan T, Fujimoto K, Qayyum A (2010) Chronic hepatitis and cirrhosis on MR imaging. Magn Reson Imaging Clin N Am 18:383–402
Ito K, Mitchell DG, Gabata T, Hussain SM (1999) Expanded gallbladder fossa: simple MR imaging sign of cirrhosis. Radiology 211:723–726
Ito K, Mitchell DG, Kim MJ, Awaya H, Koike S, Matsunaga N (2003) Right posterior hepatic notch sign: a simple diagnostic MR finding of cirrhosis. J Magn Reson Imaging 18:561–566
Rofsky NM, Weinreb JC, Ambrosino MM, Safir J, Krinsky G (1996) Comparison between in-phase and opposed-phase T1-weighted breath-hold FLASH sequences for hepatic imaging. J Comput Assist Tomogr 20:230–235
Leiber LM, Boursier J, Michalak S et al (2015) MRI versus histological methods for time course monitoring of steatosis amount in a murine model of NAFLD. Diagn Interv Imaging 96:915–922
Soyer P, Dufresne AC, Somveille E, Scherrer A (1996) Focal nodular hyperplasia of the liver: assessment of hemodynamic and angioarchitectural patterns with gadolinium chelate-enhanced 3D spoiled gradient-recalled MRI and maximum intensity projection reformatted images. J Comput Assist Tomogr 20:898–904
Barral M, Sirol M, Placé V et al (2012) Hepatic and pancreatic involvement in hereditary hemorrhagic telangiectasia: quantitative and qualitative evaluation with 64-section CT in asymptomatic adult patients. Eur Radiol 22:161–170
Ravard G, Soyer P, Boudiaf M et al (2004) Hepatic involvement in hereditary hemorrhagic telangiectasia: helical computed tomography features in 24 consecutive patients. J Comput Assist Tomogr 28:488–495
Naganuma H, Ishida H, Niizawa M, Igarashi K, Shioya T, Masamune O (1995) Hepatic involvement in Osler-Weber-Rendu disease: findings on pulsed and color Doppler sonography. AJR Am J Roentgenol 165:1421–1425
Waguri N, Suda T, Kamura T, Aoyagi Y (2002) Heterogeneous hepatic enhancement on CT angiography in idiopathic portal hypertension. Liver 22:276–280
Yoshida M, Nakaura T, Inoue T et al (2018) Magnetic resonance cholangiopancreatography with GRASE sequence at 3.0T: does it improve image quality and acquisition time as compared with 3D TSE? Eur Radiol 28:2436–2443
Nam JG, Lee JM, Kang HJ et al (2018) GRASE revisited: breath-hold three-dimensional (3D) magnetic resonance cholangiopancreatography using a gradient and spin echo (GRASE) technique at 3T. Eur Radiol 28:3721–3728
Hocquelet A, Frulio N, Gallo G et al (2018) Point-shear wave elastography predicts liver hypertrophy after portal vein embolization and postoperative liver failure. Diagn Interv Imaging 99:371–379
Funding
This research received no financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Philippe Soyer.
Conflict of interest
The authors have no conflicts of interest to disclose regarding this study.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 52 kb)
Rights and permissions
About this article
Cite this article
Chouraqui, E., Leguilloux, L., Dohan, A. et al. Can we differentiate HIV-associated obliterative portopathy from liver cirrhosis using MRI?. Eur Radiol 30, 213–223 (2020). https://doi.org/10.1007/s00330-019-06391-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06391-6