Abstract
Objective
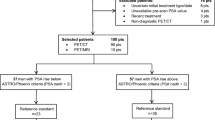
To investigate the diagnostic potential of simultaneous 18F-fluciclovine PET/MRI for pelvic lymph node (LN) staging in patients with high-risk prostate cancer.
Methods
High-risk prostate cancer patients (n=28) underwent simultaneous 18F-fluciclovine PET/MRI prior to surgery. LNs were removed according to a predefined template of eight regions. PET and MR images were evaluated for presence of LN metastases according to these regions. Sensitivity/specificity for detection of LN metastases were calculated on patient and region basis. Sizes of LN metastases in regions with positive and negative imaging findings were compared with linear mixed models. Clinical parameters of PET-positive and -negative stage N1 patients were compared with the Mann-Whitney U test.
Results
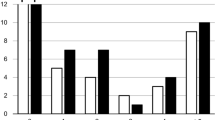
Patient- and region-based sensitivity/specificity for detection of pelvic LN metastases was 40 %/87.5 % and 35 %/95.7 %, respectively, for MRI and 40 %/100 % and 30 %/100 %, respectively, for PET. LN metastases in true-positive regions were significantly larger than metastases in false-negative regions. PET-positive stage N1 patients had higher metastatic burden than PET-negative N1 patients.
Conclusion
Simultaneous 18F-fluciclovine PET/MRI provides high specificity but low sensitivity for detection of LN metastases in high-risk prostate cancer patients. 18F-Fluciclovine PET/MRI scan positive for LN metastases indicates higher metastatic burden than negative scan.
Key Points
• 18F-Fluciclovine PET/MRI has high specificity for detection of lymph node metastasis.
• 18F-Fluciclovine PET/MRI lacks sensitivity to replace ePLND.
• 18F-Fluciclovine PET/MRI may be used to aid surgery and select adjuvant therapy.
• 18F-Fluciclovine PET-positive patients have more extensive disease than PET-negative patients.
• Size of metastatic lymph nodes is an important factor for detection.




Similar content being viewed by others
Abbreviations
- AC:
-
Attenuation correction
- BP1:
-
Bed position 1
- BP2:
-
Bed position 2
- CT:
-
Computed tomography
- DCE:
-
Dynamic contrast-enhanced
- DWI:
-
Diffusion-weighted imaging
- ePLND:
-
Extended pelvic LN dissection
- FN:
-
False negative
- FP:
-
False positive
- GS:
-
Gleason score
- ITD:
-
Index tumour diameter
- LN:
-
Lymph node
- MRI:
-
Magnetic resonance imaging
- MRSI:
-
Magnetic resonance spectroscopic imaging
- NPV:
-
Negative predictive value
- PET:
-
Positron emission tomography
- PPV:
-
Positive-predictive value
- PRESS:
-
Point RESolved Spectroscopy
- RARP:
-
Robot-assisted radical prostatectomy
- SPACE:
-
Sampling perfection with application-optimized contrasts using different flip angle evolution
- SS-EPI:
-
Single-shot echo planar imaging
- T1W:
-
T1-weighted
- T2W:
-
T2-weighted
- TE:
-
Echo time
- TN:
-
True negative
- TP:
-
True positive
- TR:
-
Repetition time
- TSE:
-
Turbo spin cho
- VIBE:
-
Volume interpolated gradient echo
References
Hovels AM, Heesakkers RA, Adang EM et al (2008) The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol 63:387–395
Heidenreich A, Bastian PJ, Bellmunt J et al (2014) EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 65:124–137
Briganti A, Chun FK, Salonia A et al (2006) Complications and other surgical outcomes associated with extended pelvic lymphadenectomy in men with localized prostate cancer. Eur Urol 50:1006–1013
Sankineni S, Brown AM, Fascelli M et al (2015) Lymph node staging in prostate cancer. Curr Urol Rep 16:30
Schuster DM, Nieh PT, Jani AB et al (2014) Anti-3-[(18)F]FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol 191:1446–1453
Turkbey B, Mena E, Shih J et al (2014) Localized prostate cancer detection with 18F FACBC PET/CT: comparison with MR imaging and histopathologic analysis. Radiology 270:849–856
Poulsen MH, Bouchelouche K, Hoilund-Carlsen PF et al (2012) [18F]fluoromethylcholine (FCH) positron emission tomography/computed tomography (PET/CT) for lymph node staging of prostate cancer: a prospective study of 210 patients. BJU Int 110:1666–1671
Beheshti M, Imamovic L, Broinger G et al (2010) 18F choline PET/CT in the preoperative staging of prostate cancer in patients with intermediate or high risk of extracapsular disease: a prospective study of 130 patients. Radiology 254:925–933
Maurer T, Gschwend JE, Rauscher I et al (2016) Diagnostic Efficacy of (68)Gallium-PSMA Positron Emission Tomography Compared to Conventional Imaging for Lymph Node Staging of 130 Consecutive Patients with Intermediate to High Risk Prostate Cancer. J Urol 195:1436–1443
Odewole OA, Tade FI, Nieh PT et al (2016) Recurrent prostate cancer detection with anti-3-[(18)F]FACBC PET/CT: comparison with CT. Eur J Nucl Med Mol Imaging 43:1773–1783
Nanni C, Zanoni L, Pultrone C et al (2016) (18)F-FACBC (anti1-amino-3-(18)F-fluorocyclobutane-1-carboxylic acid) versus (11)C-choline PET/CT in prostate cancer relapse: results of a prospective trial. Eur J Nucl Med Mol Imaging 43:1601–1610
Oka S, Okudaira H, Yoshida Y, Schuster DM, Goodman MM, Shirakami Y (2012) Transport mechanisms of trans-1-amino-3-fluoro[1-(14)C]cyclobutanecarboxylic acid in prostate cancer cells. Nucl Med Biol 39:109–119
Okudaira H, Nakanishi T, Oka S et al (2013) Kinetic analyses of trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid transport in Xenopus laevis oocytes expressing human ASCT2 and SNAT2. Nucl Med Biol 40:670–675
Sorensen J, Owenius R, Lax M, Johansson S (2013) Regional distribution and kinetics of [18F]fluciclovine (anti-[18F]FACBC), a tracer of amino acid transport, in subjects with primary prostate cancer. Eur J Nucl Med Mol Imaging 40:394–402
Elschot M, Selnaes KM, Sandsmark E et al (2017) A PET/MRI study towards finding the optimal [18F]Fluciclovine PET protocol for detection and characterisation of primary prostate cancer. Eur J Nucl Med Mol Imaging 44:695–703
(2016) FDA Approves 18F-Fluciclovine and 68Ga-DOTATATE Products. J Nucl Med 57:9N
Schuster DM, Votaw JR, Nieh PT et al (2007) Initial experience with the radiotracer anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J Nucl Med 48:56–63
Suzuki H, Inoue Y, Fujimoto H et al (2016) Diagnostic performance and safety of NMK36 (trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid)-PET/CT in primary prostate cancer: multicenter Phase IIb clinical trial. Jpn J Clin Oncol 46:152–162
Inoue Y, Asano Y, Satoh T et al (2014) Phase IIa Clinical Trial of Trans-1-Amino-3-(18)F-Fluoro-Cyclobutane Carboxylic Acid in Metastatic Prostate Cancer. Asia Ocean J Nucl Med Biol 2:87–94
D'Amico AV, Whittington R, Malkowicz SB et al (1998) Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280:969–974
Joniau S, Van den Bergh L, Lerut E et al (2013) Mapping of pelvic lymph node metastases in prostate cancer. Eur Urol 63:450–458
Cardillo G (2006) Clinical test performance: the performance of a clinical test based on the Bayes theorem
Soret M, Bacharach SL, Buvat I (2007) Partial-volume effect in PET tumor imaging. J Nucl Med 48:932–945
Axumin (fluciclovine F 18) Injection, Printed Labeling Available via https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208054Orig1s000Lbl.pdf
Lyons K, Seghers V, Sorensen JI et al (2015) Comparison of Standardized Uptake Values in Normal Structures Between PET/CT and PET/MRI in a Tertiary Pediatric Hospital: A Prospective Study. AJR Am J Roentgenol 205:1094–1101
Schiavina R, Scattoni V, Castellucci P et al (2008) 11C-choline positron emission tomography/computerized tomography for preoperative lymph-node staging in intermediate-risk and high-risk prostate cancer: comparison with clinical staging nomograms. Eur Urol 54:392–401
Steuber T, Schlomm T, Heinzer H et al (2010) [F(18)]-fluoroethylcholine combined in-line PET-CT scan for detection of lymph-node metastasis in high risk prostate cancer patients prior to radical prostatectomy: Preliminary results from a prospective histology-based study. Eur J Cancer 46:449–455
van Leeuwen PJ, Emmett L, Ho B et al (2017) Prospective evaluation of 68Gallium-prostate-specific membrane antigen positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer. BJU Int 119:209–215
Reyes DK, Pienta KJ (2015) The biology and treatment of oligometastatic cancer. Oncotarget 6:8491–8524
Engel J, Bastian PJ, Baur H et al (2010) Survival benefit of radical prostatectomy in lymph node-positive patients with prostate cancer. Eur Urol 57:754–761
Gakis G, Boorjian SA, Briganti A et al (2014) The role of radical prostatectomy and lymph node dissection in lymph node-positive prostate cancer: a systematic review of the literature. Eur Urol 66:191–199
Delso G, Furst S, Jakoby B et al (2011) Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. J Nucl Med 52:1914–1922
Briganti A, Karnes JR, Da Pozzo LF et al (2009) Two positive nodes represent a significant cut-off value for cancer specific survival in patients with node positive prostate cancer. A new proposal based on a two-institution experience on 703 consecutive N+ patients treated with radical prostatectomy, extended pelvic lymph node dissection and adjuvant therapy. Eur Urol 55:261–270
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Tone F. Bathen, NTNU – Norwegian University of Science and Technology, Faculty of Medicine and Health Sciences.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Funding
This study has received funding by ‘The Norwegian Cancer Society’ and ‘The Liaison Committee between the Central Norway Regional Health Authority (RHA) and NTNU’.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in Elschot, M., et al., A PET/MRI study towards finding the optimal [18F]Fluciclovine PET protocol for detection and characterisation of primary prostate cancer. Eur J Nucl Med Mol Imaging, 2017
Methodology
• prospective
• diagnostic or prognostic study
• performed at one institution
Electronic supplementary material
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Selnæs, K.M., Krüger-Stokke, B., Elschot, M. et al. 18F-Fluciclovine PET/MRI for preoperative lymph node staging in high-risk prostate cancer patients. Eur Radiol 28, 3151–3159 (2018). https://doi.org/10.1007/s00330-017-5213-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5213-1