Abstract
Objectives
To assess structural and functional changes of the amygdala due to premenstrual syndrome (PMS) using magnetic resonance imaging (MRI).
Methods
Twenty PMS patients and 21 healthy control (HC) subjects underwent a 6-min resting-state fMRI scan during the luteal phase as well as scanning high-resolution T1-weighted images. Subcortical amygdala-related volume and functional connectivity (FC) were estimated between the two groups. Each subject completed a daily record of severity of problems (DRSP) to measure the severity of clinical symptoms.
Results
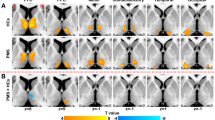
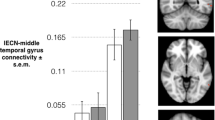
Greater bilateral amygdalae volumes were found in PMS patients compared with HC subjects, and PMS patients had increased FC between the amygdala and certain regions of the frontal cortex (e.g. medial prefrontal cortex (mPFC), anterior cingulate cortex (ACC), right precentral gyrus), the right temporal pole and the insula, as well as decreased FC between the bilateral amygdalae and the right orbitofrontal cortex and right hippocampus. The strength of FC between the right amygdala and right precentral gyrus, left ACC and left mPFC were significantly and positively correlated with DRSP scores in PMS patients.
Conclusions
Our findings may improve our understanding of the neural mechanisms involved in PMS.
Key Points
• Functional and structural MRI used to explore amygdala in PMS patients.
• Aberrant amygdala structural and functional connectivity were found in PMS patients.
• Amygdala strength FC was positively correlated with individual clinical symptom scores.



Similar content being viewed by others
Abbreviations
- ACC:
-
Anterior cingulate cortex
- AN:
-
Affective network
- BMI:
-
Body mass index
- BOLD:
-
Blood oxygenation level dependent
- DMN:
-
Default mode network
- DRSP:
-
Daily record of severity of problems
- DSM-5:
-
Diagnostic and Statistical Manual of Mental Disorders-5th Edition
- EPI:
-
Echo planar imaging
- fMRI:
-
Functional magnetic resonance imaging
- FOV:
-
Field of view
- HC:
-
Healthy control
- HIPP:
-
Hippocampus
- mPFC:
-
Medial prefrontal cortex
- OFC:
-
Orbitofrontal cortex
- PMDD:
-
Premenstrual dysphoric disorder
- PMS:
-
Premenstrual syndrome
- ROI:
-
Region of interest
- rs-fMRI:
-
Resting-state functional magnetic resonance imaging
- TE:
-
Echo time
- TR:
-
Repetition time
References
Lisofsky N, Lindenberger U, Kuhn S (2015) Amygdala/hippocampal activation during the menstrual cycle: evidence for lateralization of effects across different tasks. Neuropsychologia 67:55–62
Ossewaarde L, van Wingen GA, Rijpkema M et al (2013) Menstrual cycle-related changes in amygdala morphology are associated with changes in stress sensitivity. Hum Brain Mapp 34:1187–1193
Sundstrom Poromaa I, Gingnell M (2014) Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Front Neurosci 8:380
Cooke BM, Breedlove SM, Jordan CL (2003) Both estrogen receptors and androgen receptors contribute to testosterone-induced changes in the morphology of the medial amygdala and sexual arousal in male rats. Horm Behav 43:336–346
Greco B, Allegretto EA, Tetel MJ et al (2001) Coexpression of ER beta with ER alpha and progestin receptor proteins in the female rat forebrain: effects of estradiol treatment. Endocrinology 142:5172–5181
Bayer J, Schultz H, Gamer M et al (2014) Menstrual-cycle dependent fluctuations in ovarian hormones affect emotional memory. Neurobiol Learn Mem 110:55–63
Ryu A, Kim TH (2015) Premenstrual syndrome: A mini review. Maturitas 82:436–440
Tolossa FW, Bekele ML (2014) Prevalence, impacts and medical managements of premenstrual syndrome among female students: cross-sectional study in College of Health Sciences, Mekelle University, Mekelle, northern Ethiopia. BMC Womens Health 14:52
Yonkers KA, O'Brien PM, Eriksson E (2008) Premenstrual syndrome. Lancet 371:1200–1210
Halbreich U (2003) The etiology, biology, and evolving pathology of premenstrual syndromes. Psychoneuroendocrinology 28:55–99
Simons LE, Moulton EA, Linnman C et al (2014) The human amygdala and pain: evidence from neuroimaging. Hum Brain Mapp 35:527–538
Etkin A, Wager TD (2007) Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164:1476–1488
Murray EA, Wise SP, Drevets WC (2011) Localization of dysfunction in major depressive disorder: prefrontal cortex and amygdala. Biol Psychiatry 69:e43–e54
Bornhovd K, Quante M, Glauche V et al (2002) Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain 125:1326–1336
Protopopescu X, Tuescher O, Pan H et al (2008) Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord 108:87–94
Neugebauer V (2007) The amygdala: different pains, different mechanisms. Pain 127:1–2
Greicius MD, Flores BH, Menon V et al (2007) Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62:429–437
Hutton C, Draganski B, Ashburner J et al (2009) A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage 48:371–380
Liu Q, Li R, Zhou R et al (2015) Abnormal Resting-State Connectivity at Functional MRI in Women with Premenstrual Syndrome. PLoS One 10:e0136029
Du M, Liu J, Chen Z et al (2014) Brain grey matter volume alterations in late-life depression. J Psychiatry Neurosci 39:397–406
Talati A, Pantazatos SP, Schneier FR et al (2013) Gray matter abnormalities in social anxiety disorder: primary, replication, and specificity studies. Biol Psychiatry 73:75–84
Jeong HG, Ham BJ, Yeo HB et al (2012) Gray matter abnormalities in patients with premenstrual dysphoric disorder: an optimized voxel-based morphometry. J Affect Disord 140:260–267
Endicott J, Nee J, Harrison W (2006) Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health 9:41–49
Shehata NA (2016) Calcium versus oral contraceptive pills containing drospirenone for the treatment of mild to moderate premenstrual syndrome: a double blind randomized placebo controlled trial. Eur J Obstet Gynecol Reprod Biol 198:100–104
Halbreich U, Backstrom T, Eriksson E et al (2007) Clinical diagnostic criteria for premenstrual syndrome and guidelines for their quantification for research studies. Gynecol Endocrinol 23:123–130
Us APAAV (2013) Desk reference to the diagnostic criteria from DSM-5™. American Psychiatric Publishing 2013:7
Bao AM, Ji YF, Van Someren EJ et al (2004) Diurnal rhythms of free estradiol and cortisol during the normal menstrual cycle in women with major depression. Horm Behav 45:93–102
Yuan K, Zhao L, Cheng P et al (2013) Altered structure and resting-state functional connectivity of the basal ganglia in migraine patients without aura. J Pain 14:836–844
Hermel EE, Ilha J, Xavier LL et al (2006) Influence of sex and estrous cycle, but not laterality, on the neuronal somatic volume of the posterodorsal medial amygdala of rats. Neurosci Lett 405:153–158
McEwen BS, Magarinos AM, Reagan LP (2002) Studies of hormone action in the hippocampal formation: possible relevance to depression and diabetes. J Psychosom Res 53:883–890
Drevets WC, Price JL, Furey ML (2008) Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 213:93–118
Frodl T, Meisenzahl E, Zetzsche T et al (2002) Enlargement of the amygdala in patients with a first episode of major depression. Biol Psychiatry 51:708–714
Cooke BM (2006) Steroid-dependent plasticity in the medial amygdala. Neuroscience 138:997–1005
Gusnard DA, Raichle ME, Raichle ME (2001) Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2:685–694
Ji G, Sun H, Fu Y et al (2010) Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J Neurosci 30:5451–5464
Davidson RJ (2002) Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry 51:68–80
Lenze EJ (2003) Comorbidity of depression and anxiety in the elderly. Curr Psychiatry Rep 5:62–67
Zhuo M (2016) Contribution of synaptic plasticity in the insular cortex to chronic pain. Neuroscience 338:220–229
Chen Z, Chen X, Liu M et al (2017) Altered functional connectivity of amygdala underlying the neuromechanism of migraine pathogenesis. J Headache Pain 18:7
Schweinhardt P, Bushnell MC (2010) Pain imaging in health and disease--how far have we come? J Clin Invest 120:3788–3797
Martucci KT, Mackey SC (2016) Imaging Pain. Anesthesiol Clin 34:255–269
Baur V, Hanggi J, Langer N et al (2013) Resting-state functional and structural connectivity within an insula-amygdala route specifically index state and trait anxiety. Biol Psychiatry 73:85–92
Mufson EJ, Mesulam MM, Pandya DN (1981) Insular interconnections with the amygdala in the rhesus monkey. Neuroscience 6:1231–1248
Lesting J, Narayanan RT, Kluge C et al (2011) Patterns of coupled theta activity in amygdala-hippocampal-prefrontal cortical circuits during fear extinction. PLoS One 6:e21714
Etkin A, Egner T, Kalisch R (2011) Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 15:85–93
Toazza R, Franco AR, Buchweitz A et al (2016) Amygdala-based intrinsic functional connectivity and anxiety disorders in adolescents and young adults. Psychiatry Res 257:11–16
Funding
The present study was supported by the Guangxi Natural Science Foundation [Grant No. 2017JJB10213, 2016GXNSFAA380086, 2011GXNSFA018176] and National Natural Science Foundation of China [Grant No. 81760886, 81471738, 81303060].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Demao Deng.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Informed consent
All participants were informed about the experimental procedure and provided written informed consent.
Ethical approval
The study was approved by the Medicine Ethics Committee of First Affiliated Hospital, Guangxi University of Chinese Medicine, Guangxi, China.
Methodology
• prospective
• case-control study
• performed at one institution
Electronic supplementary material
ESM 1
(DOC 74 kb)
Rights and permissions
About this article
Cite this article
Deng, D., Pang, Y., Duan, G. et al. Larger volume and different functional connectivity of the amygdala in women with premenstrual syndrome. Eur Radiol 28, 1900–1908 (2018). https://doi.org/10.1007/s00330-017-5206-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5206-0