Abstract
Objective
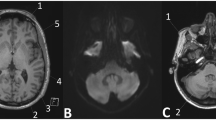
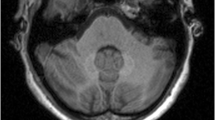
To evaluate correlation between cumulative dose of gadobutrol and signal intensity (SI) within dentate nucleus and globus pallidus on unenhanced T1-weighted images in patients with relapsing-remitting multiple sclerosis (RRMS).
Methods
Dentate nucleus-to-pons and globus pallidus-to-thalamus SI ratios, and renal and liver functions, were evaluated after multiple intravenous administrations of 0.1 mmol/kg gadobutrol at 27, 96–98, and 168 weeks. We compared SI ratios based on the number of administrations, total amount of gadobutrol administered, and time between injections.
Results
Globus pallidus-to-thalamus (p = 0.025) and dentate nucleus-to-pons (p < 0.001) SI ratios increased after multiple gadobutrol administrations, correlated with the number of administrations (ρ = 0.263, p = 0.046, respectively) and depended on the length of administration (p = 0.017, p = 0.037, respectively). Patients receiving gadobutrol at 27 weeks showed the greatest increase in both SI ratios (p = 0.006; p = 0.014, respectively, versus 96–98 weeks). GGT increased at the end of the study (p = 0.004).
Conclusion
In patients with RRMS, SI within the dentate nucleus and globus pallidus increased on unenhanced T1-weighted images after multiple gadobutrol injections. Administration of the same total amount of gadobutrol over a shorter period caused greater SI increase.
Key points
• Gadolinium deposition may occur within the human brain after multiple gadolinium contrast administrations
• Increasing T1W signal intensity occurs within the dentate nucleus and globus pallidus
• Increasing signal intensity may be a consequence of multiple administrations of gadobutrol
• Administration of gadobutrol over a shorter period causes greater signal intensity increase




Similar content being viewed by others
Abbreviations
- MRI:
-
Magnetic resonance imaging
- Gd:
-
Gadolinium
- SI:
-
Signal intensity
- RRMS:
-
Relapsing-remitting multiple sclerosis
- SPMS:
-
Secondary progressive multiple sclerosis
References
Giesel F, Mehndiratta A, Essig M (2010) High-relaxivity contrast enhanced magnetic resonance neuroimaging: a review. Eur Radiol 20:2461–2474
Caravan P, Ellison JJ, Mcmurry TJ, Lauferr JB (1999) Gadolinium (III) Chelates as MRI contrast agents: structures, dynamics and applications. Chem Rev 99:2293–2352
Bellin MF, Vasile M, Morel-Prtecetti S (2003) Currently used non-specific extracellular MR contrast media. Eur Radiol 13:2688–2698
Pietsch H, Lengsfeld P, Jost G et al (2009) Long-term retention of gadolinium in the skin of following the administration of gadolinium based contrast agents. Eur Radiol 19:1417–1424
Sieber MA, Pietsch H, Walter J et al (2008) A preclinical study to investigate the development of Nephrogenic Systemic Fibrosis: a possible role for gadolinium-based contrast media. Investig Radiol 43:65–75
Sieber MA, Pietsch H, Walter J et al (2008) Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in-vivo and to trigger nephrogenic systemic fibrosis-like lesions. Eur Radiol 18:2164–2173
Kanda T, Ishii K, Kawaguchi K et al (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834–841
Roccatagliata L, Vuolo L, Bonzano L et al (2009) Multiple sclerosis: hyperintense dentate nucleus on unenhanced T1-weighted images is associated with the secondary progressive subtype. Radiology 251:503–510
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revision to the Mc Donald criteria. Ann Neurol 69:292–302
Kasahara S, Miki Y, Kanagaki M et al (2011) Hyperintense dentate nucleus on unenhanced T1-weighted MR images is associated with a history of brain irradiation. Radiology 258:222–258
LeVine SM (1997) Iron deposits in multiple sclerosis and Alzheimer’s disease brains. Brain Res 760:298–303
Chan DE, Pan HC, Ho DM et al (2007) Presence of activated microglia in a high-signal lesion on T1-weighted MR images: a biopsy sample re-examined. AJNR Am J Neuroradiol 28:602
Drayer B, Burger P, Hurwitz B et al (1987) Reduced signal intensity on MR images of thalamus and putamen in multiple sclerosis: increased iron content? AJR Am J Roentgenol 149:357–363
Brass SD, Chen NK, Mulkern NV, Bakshi R (2006) Magnetic resonance imaging of iron deposition in neurological disorders. Top Magn Reson Imaging 17:31–40
Drayer BP, Burger P, Hurwtz B et al (1987) Magnetic resonance imaging in multiple sclerosis: decreased signal in thalamus and putamen. Ann Neurol 22:546–550
Craelius W, Migdal MW, Luessenhop CP et al (1982) Iron deposits surrounding multiple sclerosis plaques. Arch Pathol Lab Med 106:397–399
Shin JC, Kim E, Sheong HK et al (2007) High signal intensity on magnetic resonance imaging as a predictor of neurobehavioral performance of workers exposed to manganese. Neurotoxicology 28:257–262
Fujioka M, Taoka T, Matsuo Y et al (2003) Magnetic resonance imaging shows delayed ischemic striatal neurodegeneration. Ann Neurol 54:732–747
Powell T, Sussman JG, Davies-Jones GA (1992) MR imaging in acute multiple sclerosis: ring-like appearance in plaque suggesting the presence of paramagnetic free radicals. AJNR Am J Neuroradiol 13:1544–1546
Terada H, Barkovich AJ, Edwards MS, Ciricillo SM (1996) Evolution of high-intensity basal ganglia lesions on T1-weighted MR in neurofibromatosis type 1. AJNR Am J Neuroradiol 17:755–760
Daszkiewicz OK, Hennel JW, Szczepkowski TW, Lubas B (1963) Proton magnetic relaxation and protein hydration. Nature 200:1006–1007
Henkelman RM, Watts JF, Kucharzyk W (1991) High signal intensity in MR images of calcified brain tissue. Radiology 179:199–206
Boyko OB, Burger PC, Shelburne JD, Ingram P (1992) Non-heme mechanisms for T1 shortening: pathologic, CT, and MR elucidation. AJNR Am J Neuroradiol 13:1439–1445
Warakaulle DR, Anslow P (2003) Differential diagnosis of intracranial lesions with high signal on T1 or low signal on T-2 weighted MRI. Clin Radiol 58:922–933
Suzuki S, Nishio S, Takata K et al (2000) Radiation-induced brain calcification: paradoxical high signal intensity in T1-weighted images. Acta Neurochir (Wien) 142:801–804
Weinmann HJ, Gries H, Speck U (1992) Fundamental physics and chemistry: Types of contrast agents. In: Sartor K (ed) MR imaging of the skull and brain: a correlative text atlas. Springer-Verlag, New York, NY, pp 26–28
Hegde A, Mohan S, Lath N, Lim CCT (2011) Differential diagnosis for bilateral abnormalities of basal ganglia and thalamus. Radiographics 31:5–30
Kim TJ, Kim TO, Kim WS et al (2006) MR imaging of the brain in Wilson disease of childhood: findings before and after treatment with clinical correlation. AJNR Am J Neuroradiol 2:1373–1378
Lai PH, Chen C, Liang HL, Pan HB (1999) Hyperintense basal ganglia on T1-weighted MR imaging. AJNR Am J Neuroradiol 172:1109–1115
Valdés Hernández Mdel C, Maconick LC, Tan EM, Wardlaw JM (2012) Identification of mineral deposits in the brain on radiological images: a systematic review. Eur Radiol 22:2371–2381
Brunberg JA, Kanal E, Hirsch W, Van Thiel DH (1991) Chronic acquired hepatic failure: MR imaging of the brain at 1.5 T. AJNR Am J Neuroradiol 12:909–914
Rovira A, Alonso J, Cordoba J (2008) MR imaging findings in hepatic encephalopathy. AJNR Am J Neuroradiol 29:1612–1621
Oikonomu A, Chatzistefanou A, Zezos P et al (2012) Basal ganglia hyperintensity on T1-weighted MRI in Rendu-Osler-Weber disease. J Magn Reson Imaging 35:426–430
Mirowitz SA, Westicks TJ, Hirsch JD (1991) Hyperdense basal ganglia on T1-weighted images in patients receiving parenteral nutrition. Radiology 181:117–120
da Silva CJ, da Rocha AJ, Jeronymo S et al (2007) A preliminary study revealing a new association in patients undergoing maintenance hemodialysis: manganism symptoms and T1 hyperintense changes in the basal ganglia. AJNR Am J Neuroradiol 28:1474–1479
Martin-Duverneuil N, Idbaih A, Hoang-Xuan K et al (2006) MRI features of neurodegenerative Langerhans cell histiocytosis. Eur Radiol 16:2074–2082
White GW, Gibby WA, Tweedle MF (2006) Comparison of Gd(DTPA-BMA) (Omniscan) versus Gd(HP-D03A) (ProHance) relative to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Investig Radiol 41:272–278
Shellock FG, Kanal E (1999) Safety of magnetic resonance imaging contrast agents. J Magn Reson Imaging 10:477–484
Tweedle MF, Wedeking P, Krishan K (1995) Biodistribution of radiolabeled, formulated gadopentenate, gadoteridol, gadoterate, and gadodiamide in mice and rats. Investig Radiol 30:371–380
Acknowledgments
The scientific guarantor of this publication is Prof. Dr. Dragan Stojanov The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors has significant statistical expertise. No complex statistical methods were necessary for this paper. Institutional Review Board approval and written informed consent were not required because the retrospective nature of our clinically acquired data. At the time of the examination, however, all patients had given consent to use their clinical and imaging data for research. Study subjects or cohorts have not been previously reported. Methodology: prospective / retrospective.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stojanov, D.A., Aracki-Trenkic, A., Vojinovic, S. et al. Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur Radiol 26, 807–815 (2016). https://doi.org/10.1007/s00330-015-3879-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3879-9