Abstract
Objective
To evaluate the feasibility of in vivo 23Na imaging of the corticomedullary 23Na gradient and to measure 23Na transverse relaxation times (T2*) in human kidneys.
Methods
In this prospective, IRB-approved study, eight healthy volunteers (4 female, 4 male; mean age 29.4 ± 3.6 years) were examined on a 7-T whole-body MR system using a 23Na-only spine-array coil. For morphological 23Na-MRI, a 3D gradient echo (GRE) sequence with a variable echo time scheme (vTE) was used. T2* times were calculated using a multiecho 3D vTE-GRE approach. 23Na signal-to-noise ratios (SNR) were given on a pixel-by-pixel basis for a 20-mm section from the cortex in the direction of the medulla. T2* maps were calculated by fitting the 23Na signal decay monoexponentially on a pixel-by-pixel basis, using least squares fit.
Results
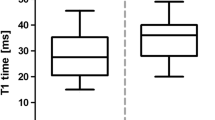
Mean corticomedullary 23Na-SNR increased from the cortex (32.2 ± 5.6) towards the medulla (85.7 ± 16.0). The SNR increase ranged interindividually from 57.2 % to 66.3 %. Mean 23Na-T2* relaxation times differed statistically significantly (P < 0.001) between the cortex (17.9 ± 0.8 ms) and medulla (20.6 ± 1.0 ms).
Conclusion
The aim of this study was to evaluate the feasibility of in vivo 23Na MRI of the corticomedullary 23Na gradient and to measure the 23Na T2* relaxation times of human kidneys at 7 T.
Key Points
• High field MR offers new insights into renal anatomy and physiology.
• 23 Na MRI of healthy human kidneys is feasible at ultra-high field.
• Renal 23 Na concentration increases from the cortex in the medullary pyramid direction.
• In vivo measurements of renal 23 Na-T2* times are demonstrated at 7.0 T.





Similar content being viewed by others
References
Xu X, Fang W, Ling H et al (2010) Diffusion-weighted MR imaging of kidneys in patients with chronic kidney disease: Initial study. Eur Radiol 20:978–983
Michaely HJ, Metzger L, Haneder S et al (2012) Renal BOLD-MRI does not reflect renal function in chronic kidney disease. Kidney Int 81:684–689
Li LP, Halter S, Prasad PV (2008) Blood oxygen level-dependent MR imaging of the kidneys. Magn Reson Imaging Clin N Am 16:613–625
Maril N, Margalit R, Rosen S et al (2006) Detection of evolving acute tubular necrosis with renal 23Na MRI: Studies in rats. Kidney Int 69:765–768
Maril N, Margalit R, Mispelter J et al (2004) Functional sodium magnetic resonance imaging of the intact rat kidney. Kidney Int 65:927–935
Maril N, Rosen Y, Reynolds GH et al (2006) Sodium MRI of the human kidney at 3 Tesla. Magn Reson Med 56:1229–1234
Haneder S, Konstandin S, Morelli JN et al (2011) Quantitative and qualitative 23Na MR imaging of the human kidneys at 3 T: Before and after a water load. Radiology 260:857–865
Wolff SD, Eng J, Berkowitz BA et al (1990) Sodium-23 nuclear magnetic resonance imaging of the rabbit kidney in vivo. Am J Physiol 258:1125–1131
Rosen Y, Lenkinski RE (2009) Sodium MRI of a human transplanted kidney. Acad Radiol 16:886–889
Neuberger T, Gulani V, Webb A (2007) Sodium renal imaging in mice at high magnetic fields. Magn Reson Med 58:1067–1071
Maril N, Margalit R, Mispelter J et al (2005) Sodium magnetic resonance imaging of diuresis: Spatial and kinetic response. Magn Reson Med 53:545–552
Staroswiecki E, Bangerter NK, Gurney PT et al (2010) In vivo sodium imaging of human patellar cartilage with a 3D cones sequence at 3 T and 7 T. J Magn Reson Imaging 32:446–451
Juras V, Zbyn S, Pressl C et al (2012) Sodium MR imaging of achilles tendinopathy at 7 T: Preliminary results. Radiology 262:199–205
Krusche-Mandl I, Schmitt B, Zak L et al (2012) Long-term results 8 years after autologous osteochondral transplantation: 7 T gagCEST and sodium magnetic resonance imaging with morphological and clinical correlation. Osteoarthritis Cartilage 20:357–363
Schmitt B, Zbyn S, Stelzeneder D et al (2011) Cartilage quality assessment by using glycosaminoglycan chemical exchange saturation transfer and (23)Na MR imaging at 7 T. Radiology 260:257–264
Trattnig S, Welsch GH, Juras V et al (2010) 23Na MR imaging at 7 T after knee matrix-associated autologous chondrocyte transplantation preliminary results. Radiology 257:175–184
Zbyn S, Stelzeneder D, Welsch GH et al (2012) Evaluation of native hyaline cartilage and repair tissue after two cartilage repair surgery techniques with 23Na MR imaging at 7 T: Initial experience. Osteoarthritis Cartilage 20:837–845
Ying K, Schmalbrock P, Clymer B (1995) Echo-time reduction for submillimeter resolution imaging with a 3D phase encode time reduced acquisition method. Magn Reson Med 33:82–87
Deligianni X, Bar P, Scheffler K et al (2012) High-resolution Fourier-encoded sub-millisecond echo time musculoskeletal imaging at 3 Tesla and 7 Tesla. Magn Reson Med doi:. doi:10.1002/mrm.24578
Maeda M, Seo Y, Murakami M et al (1990) Sodium-23 MR imaging of the kidney in guinea pig at 2.1 T, following arterial, venous, and ureteral ligation. Magn Reson Med 16:361–367
Liu W, Dahnke H, Rahmer J et al (2009) Ultrashort T2* relaxometry for quantitation of highly concentrated superparamagnetic iron oxide (SPIO) nanoparticle labeled cells. Magn Reson Med 61:761–766
Haneder S, Konstandin S, Morelli JN et al (2012) Assessment of the renal cortico-medullary 23Na-gradient using isotropic data sets. Acad Radiol 20:407–13
Acknowledgments
This study was supported by funding from the Vienna Clinical Imaging Centre (VIACLIC) project as part of the Vienna Spots of Excellence (VSOE) Program and by Siemens Healthcare. This study was supported non-financially by the Proof-of-Concept program of Euro-BioImaging.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haneder, S., Juras, V., Michaely, H.J. et al. In vivo sodium (23Na) imaging of the human kidneys at 7 T: Preliminary results. Eur Radiol 24, 494–501 (2014). https://doi.org/10.1007/s00330-013-3032-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-013-3032-6