Abstract
It is widely believed that deciduous tundra-shrub dominance is increasing in the pan-Arctic region, mainly due to rising temperature. We sampled dwarf birch (Betula nana L.) at a northeastern Siberian tundra site and used dendrochronological methods to explore the relationship between climatic variables and local shrub dominance. We found that establishment of shrub ramets was positively related to summer precipitation, which implies that the current high dominance of B. nana at our study site could be related to high summer precipitation in the period from 1960 to 1990. The results confirmed that early summer temperature is most influential to annual growth rates of B. nana. In addition, summer precipitation stimulated shrub growth in years with warm summers, suggesting that B. nana growth may be co-limited by summer moisture supply. The dual controlling role of temperature and summer precipitation on B. nana growth and establishment is important to predict future climate-driven vegetation dynamics in the Arctic tundra.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global climate change has markedly affected the ecosystems on Earth (ACIA 2005; IPCC 2013). Compared to other regions, the Arctic region is experiencing dramatic air temperature increase and more extreme precipitation events (Hinzman et al. 2005; McGuire et al. 2006). During the last four decades, surface air temperature increased on average 0.4 °C per decade over the Arctic (Anisimov et al. 2007).
As a consequence, local vegetation composition has changed in the North American and Scandinavian Arctic (ACIA 2005). In particular, deciduous shrubs have been observed to increase in Arctic tundra. Experimental studies in tundra ecosystems (Shaver et al. 2001; Mack et al. 2004; Walker et al. 2006; Bret-Harte et al. 2008; Nowinski et al. 2010) suggested that deciduous shrubs, especially Betula nana L. (dwarf birch), benefit from the increase in temperature, thereby becoming more dominant in the ecosystem. In the last 50 years, shrub-patch expansion was apparent at different sites throughout Alaska (Sturm et al. 2001; Tape et al. 2006), Canada (Hudson and Henry 2009) and other Arctic tundra regions (Myneni et al. 1997; Stow et al. 2004; Hudson and Henry 2009; Myers-Smith et al. 2011; Frost and Epstein 2014). The observed shrub expansion could reduce tundra albedo, leading to additional regional warming (Sturm et al. 2005; Blok et al. 2011b; Loranty et al. 2011; Juszak et al. 2014).
Field experiments, aerial photographs and remotely sensed data provided a general but coarse impression of tundra “greening” explained as shrub expansion during the last decades (Sturm et al. 2001). However, measurement errors caused by instrumentation drift, atmospheric effects (Stow et al. 2004) and low image resolution (Tape et al. 2006) limit the assessment of fine-scale shrub cover dynamics. Meanwhile, the time spans of most field warming experiments are too short to unravel the co-variation of shrub growth and local climate fluctuations (Bret-Harte et al. 2008).
Dendrochronology is a useful tool to explore the long-term relationship between climate and shrub growth and expansion (Bär et al. 2008; Myers-Smith et al. 2011, 2015b). Woody plants living under extreme conditions, such as shrubs in the Arctic, usually respond sensitively to climatic variability in their vertical and radial growth (Bradley and Jones 1992; Mäkinen et al. 2003; Myers-Smith et al. 2015a). Compared to trees, application of dendrochronology to Arctic deciduous shrubs is challenging mainly related to low growth rates and frequently missing rings (Woodcock and Bradley 1994) but also due to irregular wood formation along and around the main stem (Schweingruber et al. 2011, 2013).
Similar to other woody species in the Arctic tundra, B. nana forms exceptionally narrow annual tree rings (Groot et al. 1997; Meinardus et al. 2011; Hollesen et al. 2015). The average ring width of B. nana collected by Miller (1975) was merely 130 μm. Dendrochronological analyses of such material require application of serial sectioning, i.e., studying multiple samples along the main stem to account for frequently occurring missing rings, specifically in outer stem parts (Wilmking et al. 2012). Despite being a common circum-Arctic species that seems responsive to climate warming (Shaver et al. 2001; Wahren et al. 2005; Hollesen et al. 2015), B. nana has only recently been used for dendrochronological studies (Miller 1975; Blok et al. 2011a; Meinardus et al. 2011).
As temperature determines the length of the growing season in Arctic woody species (Walker et al. 2006; Hudson and Henry 2009; Berner et al. 2013), we assume that radial growth of B. nana at our site in the northeastern Siberian tundra is related to summer temperature. Pop et al. (2000) found that the bud break of B. nana is sensitive to spring air temperature and snowmelt. Since the growing season in the Arctic tundra is extremely short, an earlier start of the growing season usually results in increased growth. Precipitation may be crucial as well as neither waterlogged areas nor dry soil conditions favor B. nana growth (Groot et al. 1997). Precipitation could provide the main water supply for B. nana during the growing season, particularly when the shrubs grow on relatively well-drained soil or when the climate is dry. However, the growth of deciduous shrubs could also be restricted by extremely high summer precipitation due to anaerobic soil conditions that might develop (Lloyd et al. 2003). Aside from the rainfall in summer, winter precipitation (snow) can also affect shrub growth (Blok et al. 2015), as deeper snow cover in winter provides better insulation, which leads to higher turnover in soil organic matter, which is hypothesized to benefit shrub growth during the subsequent growing season (Sturm et al. 2001). The primary objectives of this study were to identify the main climatic factors that determine annual growth and establishment of B. nana, a common Arctic deciduous shrub species.
Material and methods
Site description and climate data
We collected samples of B. nana from Kytalyk nature reserve (70°49′N, 147°28′E), located in the Indigirka lowlands in northeast Siberia, Russian Federation (Fig. 1). The whole study area is underlain by continuous permafrost, and the sampling locations were in the former bed of a drained thermokarst lake. The local vegetation type is defined as G4 (tussock sedge, dwarf shrub, moss tundra) at the circumpolar Arctic Vegetation Map (Walker et al. 2005). B. nana is the most common deciduous shrub species and covers approximately 20 % of the land in the area (Blok et al. 2011a). B. nana ramets (vegetative clone) mainly occupy the palsas (permafrost hummocks), while Sphagnum mosses and Eriophorum sedges dominate the waterlogged depressions. Although B. nana shrubs produce seeds, they mainly depend on clonal growth for reproduction (Groot et al. 1997). Since the density of large herbivores is extremely low at the site, their impact on shrub growth is assumed to be minimal.
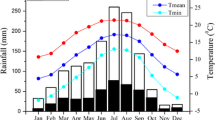
Long-term meteorological data (daily average temperature, daily cumulative precipitation and snow depth) since 1945 are available, recorded by the closest meteorological station (WMO station no. 21946 in Chokurdakh), 30 km south of the study site (http://climexp.knmi.nl/). The mean annual temperature (1981–2010) at Chokurdakh is −13.4 °C, and the mean annual precipitation is 196 mm (1981–2010) (Nauta et al. 2015). The mean July temperature of the same period is 10.3 °C. The mean temperature in January is −34.0 °C. Summer is defined as ranging from June to August, as the snow-free period usually lasts from the beginning of June until the end of August accordingly. For each year, the start of the growing season and length of the growing season were calculated using these Chokurdakh climate data.
Sample collection and preparation
In total, 90 B. nana ramets from 10 different B. nana-dominated patches (approximate 173 m2 in average) were systematically collected in July 2012. All 10 patches were located at a former thermokarst lake bed (Fig. 1), and the distance among patches was at least 30 m. In each patch, three transects (from center to patch edge) located at an angle of 120° were established. Along each transect, three ramet samples (center, middle and outside) were collected (online resource 1).
The sampling strategy in general followed the requirements for applying the serial-sectioning technique to ensure detection and measurements of all tree rings at the stem base and along the stem (Kolishchuk 1990). Individual ramet stems were cut just below the transition zone between root and stem (usually located a few centimeters below soil surface where the coarse roots started to appear; Fig. 1). After measuring the total length of a ramet, three 2- to 3-cm-thick disks were collected along the main growth axis: at the transition zone between root and stem (disk α), at the soil surface (disk β) and just below the first long branch (disk γ). All stem sections were stored immediately in glycerin–alcohol mixture (33 % glycerin, 33 % alcohol and 33 % water in volume). The samples were transported to Wageningen University for further processing.
In the Lab, a 15- to 20-µm-thick microsection was prepared from each disk of the three disks per ramet, using a GSL1 sledge microtome (Gärtner et al. 2014). For enhancing the contrast between different wood tissues which eases detection of ring boundaries, the sections were stained with a safranin/astra blue mixture. After 5-min staining, all the sections were dehydrated subsequently by using a 50, 96 and 99 % alcohol. Dehydrated samples were then washed with Roti®-Clear liquid and permanently imbedded in Roti®-Mount afterward. All the slides were eventually fastened on a steel plate by using two button-shaped magnets and air-dried for around 72 h.
Tree-ring measurement, cross-dating and chronology development
Ring widths were measured directly from the slides along at least four radii using a combination of a Lintab digital positioning table and the TSAP-Win software (both Rinntech, Germany). Ring boundaries of B. nana are usually distinctly characterized by 1–3 tangential rows of flattened fibers (Fig. 2). Since ring formation was usually not concentric, some radii include narrow rings and (partially) missing rings. Correct dating of each measured ring as well as detection of missing rings is only possible through visual cross-dating of ring-width series and—if necessary—re-inspection of the rings on the slide. This process was successively applied, first within, and then between ramets. Within a ramet, cross-dating starts on different radii of the same stem disk [α (upper main stem), β (soil surface) and γ (root/shoot transition), Fig. 2]. After calculation of a mean ring-width series for each stem disk, mean ring-width series from disks taken at the three stem heights were compared to check whether the basal ring-width series (β) contains all rings and represents the annual variation in ring width across the ramet’s lifetime. Visual cross-dating was statistically checked by applying the cross-dating quality control program COFECHA (Bunn 2008). If any missing ring was detected during the process, we manually added a v narrow ring (10 µm) at that year in the ring-width series. As missing rings turned out to be more frequent in the radii of α disks in comparison with β disks (resp. 60 and 50 % of all radii and on average 2 vs. 1.5 missing rings per radius) but both disks showed no large difference in number of detected rings, disk β was used for further analyses.
The mean ring-width series derived from the β disks were finally used to (1) determine when the individual ramets established and (2) to calculate a site chronology. For the latter, we used only individuals older than 30 years, as these are not only representing juvenile growth patterns, affected by initial competition-driven growth but mainly reflect environmental-driven growth patterns of grown up, established shrubs. Before a site chronology was calculated from these older ramets, the individual ring-width series were standardized in ARSTAN v6.05 to eliminate size- and age-related growth trends. After standardization, the index series are averaged into a site chronology (program ARSTAN) (Cook and Holmes 1986). The quality of this site chronology is checked by calculating the inter-series correlation (rbar) and the expressed signal (EPS) (Bunn 2008). Subsequently, the standardized ring-width index (RWI) was calculated. In addition, we compared the site chronology of B. nana in this study with the chronologies of B. nana and Salix pulchra Cham. in the study by Blok et al. (2011a). The RWI of B. nana (and S. pulchra) from both studies was compared as well.
Climate–growth analysis
Before analysis of climate–growth relationships, the normal distribution of each variable was checked by SPSS software (IBM SPSS Statistics for Windows, ver. 22.0; IBM Corp., Armonk, NY, USA). All variables showed a normal distribution, except some precipitation variables which were log-transformed to achieve or improve the normal distribution. Pearson’s correlation coefficients (r) were calculated for the relationships between the RWI chronology and different climatic factors: precipitation, temperature, snow depth, length of growing season (number of days with mean daily temperature over 5 °C per year) (Briffa et al. 2008) and the start date of the growing season. The start dates of the growing season during the period from 1952 to 2012 were calculated based on the equation developed by Pop et al. (2000). Parmentier et al. (2011) have calculated the start dates of the growing seasons of this region by using the same formula. In addition to precipitation, we also tested a drought index, which combines precipitation and temperature data. Standardized precipitation evapotranspiration index (SPEI, Vicente-Serrano et al. 2010) data were obtained for the study site location from the global SPEI database v2.3 (sac.csic.es/spei/database.html, Vicente-Serrano et al. 2010). Since climatic conditions can also affect next year’s radial growth (Buchwal et al. 2013), the Pearson’s correlations for the relation between RWI and the climatic variables of the preceding year were included into the analyses.
Climate–establishment analyses
For each ramet, sprouting year was based on the number of measured rings on the β disks (from soil surface). From the sprouting years of all 90 samples, we obtained the annual establishment numbers. In order to explore the relationship between climate change and shrub ramet establishment, we used the generalized linear model that takes the Poisson distribution of count data into account (in SPSS) to examine the relation between the ramet establishment number (dependent variable) and climate variables (covariate). As the year-to-year variation, particularly in precipitation, is very large and the exact ages of the samples can be underestimated due to undetected missing rings, especially in very young individuals which are difficult to cross-date, we also used 5-year sums of establishment with 5-year averages of climate data to examine relationships between establishment and climate factors.
Results
Age-related cambial growth and RWI chronology
Betula nana showed a pronounced negative logarithmic radial growth trend with increasing age (R 2 = 0.85) (online resource 2). The ring-width increment declined sharply, especially during the first 15 years (slope = 9.68 μm year−1, R 2 = 0.92), and this trend became much weaker in the later years (slope = −0.25 μm year−1, R 2 = 0.19). The results from B. nana samples from Blok et al. (2011a) showed a similar result (online resource 2). In contrast to B. nana, the growth rate of S. pulchra showed a constant negative linear trend (slope = −2.68 μm year−1, R 2 = 0.51) and ring widths were on average much larger (B. nana: 81.03 ± 38.91 μm, S. pulchra: 189.11 ± 61.59 μm) (online resource 2). Based on these 53 B. nana individuals, a final B. nana chronology was established. The chronology of B. nana was characterized by a reasonable common signal represented by rbar (0.351) and EPS (0.933). In addition, we observed that the ramet samples, which have been collected on July 20 in 2012, have final row(s) of flatted fibers (the ring boundaries of 2012).
Influence of climate factors on radial growth
Year-to-year variation in B. nana radial growth was significantly related to mean summer temperature (r = 0.47, p = 0.001, 1952–2012) (Fig. 3), but correlations with other temperature-related variables (starting date and length of growing season and previous year’s summer temperature) were not significant (p > 0.05) (Table 1). The temperature in the period from June 16 till July 20, hereafter referred to as early summer, appeared most influential (Table 1) (r = 0.727, p < 0.001, n = 50 years). The late summer temperature (July 21–August 30) had no significant effect on the shrub radial growth (p > 0.05).
Precipitation also influenced radial growth patterns, but only in the years with a warm summer (Table 1). Annual radial growth rates were significantly correlated with both current-year and previous-year summer precipitation (Table 1) when the early summer temperature was above 9.58 °C (the median during the 50 years, 1962–2011). In the years with a below median early summer temperature, only early summer temperature was significantly related to RWI and no other climatic variable (Table 1). The mean snow depth did not significantly correlate with radial growth (Table 1). The relationship between SPEI and B. nana RWI values were significant only in the years with warm summers (Table 1, r were between 0.41 and 0.51, p < 0.05). SPEI over the period previous-year June up to current-year August had the largest correlation coefficient (r = 0.51, p = 0.010, n = 25 years), with high SPEI values indicating wet climatic conditions, taking evapotranspiration into account, corresponding with high ring widths.
Influence of climate factors on ramet establishment
Most of the 90 collected B. nana ramets established 20–50 years before sampling, while only five successfully established in the last 20 years, and the 10 oldest ramets initiated growth before 1963. The increase in establishment over the 1950s and 1960s coincided with increasing summer precipitation and SPEI over the same period (Fig. 4). Similarly, the dip in establishment numbers in the late 1970s was related to negative summer SPEI values indicating dry climatic conditions (Fig. 4). Establishment numbers were low since the mid-1990s in a period of declining precipitation. The 5-year establishment numbers over the period 1951–2000 were significantly related to June–August SPEI and precipitation, but were not related to temperature or temperature-related variables (SPEI June–August: p = 0.003, AIC = 58.4; summer precipitation: p = 0.018, AIC = 61.6; early summer temperature: p = 0.143, AIC = 64.8).
Discussion
Climatic change and ramet establishment
In this study, a large number of B. nana samples were successfully cross-dated to build on a study by Blok et al. (2011a, b) on this widely spread and hence important Arctic shrub species. Although ring detection is sometimes difficult due to eccentric growth and partly missing rings, we succeeded in building a reliable B. nana site chronology with high rbar and EPS values.
The vertical growth of deciduous shrubs like B. nana can be largely restricted by local snow depth (Walker et al. 1997). Extremely low winter temperatures at our site can easily reach −30 °C. Without insulation by the snow cover, the shrub branches can hardly survive (Walker et al. 1997). Some studies in Scandinavia (Jonasson 1982; Groot et al. 1997) stated that snow cover largely influences the height of B. nana in tundra ecosystem. At our site, the maximum shrub height is approximately 30 cm (Fig. 1), which is more or less equal to the local average snow depth. In our dataset, there was a positive relationship between shrub diameter at the soil surface and height (r = 0.43, p < 0.01, n = 90, data not shown). Since the vertical and radial growth of woody species may be positively correlated (Hallinger et al. 2010), the height limitation controlled by snow depth could largely control the radial growth as well. This may explain why the ring-width growth of B. nana was initially fast, but declined quickly and stayed at very low levels after the first 15 years. Apart from the effect of snow depth, aging and competition may also influence the radial growth of B. nana. It is common in Arctic shrubs that after an initial juvenile stage, the ring widths continuously decline (Schweingruber 1996; Myers-Smith et al. 2015b). Betula nana may show a similar pattern here. Moreover, as shrub size increases, the competition for soil nutrients and light among the neighboring individuals may be more intense. More research is needed to clarify the roles of snow depth and competition on the age-related cambial growth trends (Fig. 4).
The possibility of cross-dating the ring-width series of all ramets enabled reconstruction of the dynamics of ramet establishment across shrub patches. Although the sampling strategy was not specifically designed for analyzing ramet establishment in relation to climatic variations, we did obtain a dataset of 90 aged B. nana ramets that required measuring multiple radii in 3 stem sections per ramet and use cross-dating to obtain reliable ages in this difficult species with partial missing rings. The sampling was not directed to sample the thicker ramets as the primary purpose was to investigate age distribution patterns within the shrub patches. We sampled along transects from the center to the margin of the shrub patch. There was no relationship between age and position within the transects, and also the ages of the three center positions, which were relatively close to each other, were highly variable. This lack of an age pattern suggests that we probably sampled a random sample and not specific age cohorts. However, it was striking that the temporal distribution of the ramet establishment was not random. It is clear that most ramets established in periods with high summer precipitation around 1970 and in the 1980s, while the old and young (established before 1960 or after 1995) were rare, following the significant decline in summer precipitation since 1991 (slope = 4.56 mm year−1, R 2 = 0.54, n = 20 years, n = 20 years: 1991–2010). This suggests that more ramets established when summer precipitation was high. Soil moisture supply in B. nana patches is controlled by summer precipitation and depth of permafrost thaw (Lloyd et al. 2003). As a shrub species with a highly plastic growing strategy (Bret-Harte et al. 2008), it is plausible that B. nana shrubs prefer to sprout new ramets when soil moisture is adequate, at the cost of stem growth (both radial and vertical). This may also explain the relatively low correlation between the radial growth curves and the year-to-year summer precipitation in our study. The continuously decreasing summer precipitation during the last two decades could be responsible for the low number of young ramets, i.e., recently established ramets among all samples.
We assume that the bias of missing old B. nana samples or young ramets during collection is low. Like many Arctic shrubs, B. nana shrub ramets can easily survive longer than 50 years (Groot et al. 1997). Dead stems are well preserved for years at our sites, due to the low decomposition rates of recalcitrant low-quality woody material in the harsh climate. Nevertheless, we found very few dead B. nana ramet relics inside the patches. Furthermore, for the statistical analysis we excluded the periods in which the oldest and youngest ramets established as we cannot be sure that we missed young or old ramets subjectively.
The negative precipitation trend and associated low establishment together with a lack of a positive trend in annual ring-width index could imply that there is no shrub expansion at our study site. Unfortunately, the lack of a long-term positive trend in ring-width index does not necessarily mean that climate warming did not affect shrub radial growth. The dendrochronological methods, e.g., detrending, remove gradual changes over time, as they could be the result of ontogenic, e.g., age-related, trends and optimize the inter-annual variation for investigation of growth-limiting factors. Perhaps there was a declining trend because of aging compensated by a positive trend because of climate change; it is impossible to distinguish between the two. Support for no shrub expansion comes from satellite NDVI records showing no greening trend for the study region over 2000–2010 (Blok et al. 2015). Local permafrost degradation resulting in thaw pond development and drowning of shrubs could have prevented net shrub expansion (Nauta et al. 2015). The negative precipitation trend is not unique to the study area, but also occurred in some other locations, particularly in the High Arctic (Urban et al. 2014). We used the KNMI Climate Change Atlas tool (http://climexp.knmi.nl/atlas) to generate a map of summer (JJA) precipitation trends over the years 1991–2015 for the Arctic land based on observations (CRU TS 3.22 dataset). The map (online resource 3) shows that the trends in summer precipitation are far from homogenous in the Arctic. In our study region, the Indigirka delta, the mean regression relative summer precipitation (1991–2015), is −50 % per century, and the similar patterns also exist in other Arctic regions, e.g., Lena Delta, Canadian High Arctic (online resource 3), while some other areas show strong positive trends. The large variation of trends in summer precipitation in the Arctic further suggests that shrub expansion might not happen everywhere in the Arctic tundra. However, future precipitation is expected to show an increasing trend as the retreat of sea ice results in strongly increased evaporation and precipitation (Bintanja and Selten 2014).
Climatic change and shrub growth
Several experimental studies in the Arctic (Hobbie and Chapin III 1998; Mack et al. 2004; Wahren et al. 2005; Walker et al. 2006) pointed out that the growth of B. nana was stimulated by increasing air temperature during the growing season. Most dendrochronological studies on the Arctic shrubs also indicated summer temperature as the most important factor for shrub growth through lengthening of the growing season (Hallinger et al. 2010). Myers-Smith et al. (2015a) stated that the summer temperature variable is the most influential factor that affects the variation of Arctic shrub growth. Our study confirmed that year-to-year variations in summer temperature strongly influenced the annual radial growth rates and that the mean temperature of early summer (June 16–July 20) was a more important factor for the radial growth of B. nana than that of the late growing season (Blok et al. 2011a).
Evidence from both the sample measurement and the field observation supported this deduction that early summer temperature is more important than late summer temperature for the width of a ring formed in a given year. In most ramets collected on July 20 in 2012, we observed that the final row(s) of flatted fibers were already formed, indicating that the formation of the 2012-ring was almost completed by July 20 (online resource 4). This suggests radial growth of B. nana already ceased. Furthermore, the leaf color of B. nana usually starts to turn from green to yellow and red at the beginning of August at our site (online resource 5), indicating the degradation of chlorophyll (Addicott and Lyon 1973) and lower photosynthesis activities. It further explains why the length of growing season (number of days with mean daily temperature over 5 °C per year) was not strongly correlated with the radial growth of B. nana. Although the mean temperature is usually above 5 °C until the beginning of September, from this research it seems that the radial growth of B. nana finishes approximately 3–4 weeks earlier, at both the stem base (disk α) and the upper part of the stem (disk β and disk γ) (Fig. 1).
Summer precipitation not only stimulated the sprouting of B. nana ramets, but facilitated the annual radial growth rates of B. nana shrubs as well, but only during warm summers. While low radial growth rates occur when early summer temperatures are low, relatively warm summers positively affect radial growth rates of B. nana, provided that summer precipitation, either in the actual summer and/or the previous summer, is above average. Remarkable was the peak growth rate in 2011, which coincided with a warm and wet summer, whereas the preceding summer of 2010 was even warmer but also very dry, resulting in a rather modest radial growth (online resource 2). At a nearby treeline research site, 2011 was also an exceptional year with high photosynthetic rate (Liang et al. 2014). It was recently stated that apart from summer temperature, soil moisture is another important factor that largely affects the growth of shrubs in the Arctic (Myers-Smith et al. 2015a). They further suggest that the importance of soil moisture to the growth of Arctic shrubs increased during warm summers. The results of our study support this statement, due to the positive correlation between soil moisture and summer precipitation in the study region (Liang et al. 2014).
The effect of previous year’s summer precipitation on shrub growth is probably related to local soil nutrient supply. In the Arctic tundra, rapid growth of deciduous shrubs, for instance B. nana and S. pulchra, in the early growing season (late June to early July) is mainly supported by belowground nutrient storage instead of nutrient absorption at the time that the soil is still mostly frozen (Chapin III 1980; Chapin III et al. 1990), which is a common strategy among Arctic plants. Due to the extremely harsh winter environment (frozen soil and low temperature) in northeast Siberian tundra, the belowground nutrient accumulation mainly takes place during the previous growing season (Bilbrough et al. 2000). The soil nutrient absorption of the previous years is critical to the shrub growth of the next seasons. Higher soil moisture in the Arctic tundra might accelerate the permafrost thawing at B. nana-dominated patches during the growing season by increasing soil thermal conductivity. Six years of thaw depth measurements at the research site revealed that summer thaw depth was largest in 2011, which had exceptionally large summer precipitation (Nauta et al. 2015). The larger thaw depth might lead to a higher nutrient availability in deeper soil (Wahren et al. 2005). Dry soil conditions at the surface could reduce decomposition of organic matter, potentially reducing nutrient supply (Blok et al. 2015). Further studies should include investigations on soil nutrient availability. Since growth of deciduous shrubs like B. nana is highly sensitive to changes in soil nutrient availability (Marschner et al. 2007), inter-annual variations in B. nana growth may follow variations in soil nutrient availability (Shaver et al. 2001).
Conclusion
It has been widely shown that growth of deciduous shrubs like B. nana is responsive to changes in summer air temperature. This study builds on this result and further suggests that summer precipitation also influences growth rates, perhaps indirectly through soil nutrient availability; moreover, summer precipitation seems to play an important role for shrub establishment. This implies that summer precipitation can facilitate the expansion of B. nana in Arctic tundra and that the influence of precipitation dynamics on vegetation composition changes in high-latitude ecosystems should not be ignored or underestimated.
References
ACIA (2005) Arctic climate impact assessment scientific report. Cambridge University Press, Cambridge
Addicott F, Lyon J (1973) Physiological ecology of abscission. In: Kozlowski TT (ed) Shedding of plant parts. Academic Press, New York & London, pp 85–124
Anisimov OA, Lobanov VA, Reneva SA, Shiklomanov NI, Zhang T, Nelson FE (2007) Uncertainties in gridded air temperature fields and effects on predictive active layer modeling. J Geophys Res 112:261–263. doi:10.1029/2006jf000593
Bär A, Pape R, Bräuning A, Löffler J (2008) Growth-ring variations of dwarf shrubs reflect regional climate signals in alpine environments rather than topoclimatic differences. J Biogeogr 35:625–636. doi:10.1111/j.1365-2699.2007.01804.x
Berner LT, Beck PSA, Bunn AG, Goetz SJ (2013) Plant response to climate change along the forest-tundra ecotone in northeastern Siberia. Glob Change Biol 19:3449–3462. doi:10.1111/gcb.12304
Bilbrough CJ, Welker JM, Bowman WD (2000) Early spring nitrogen uptake by snow-covered plants: a comparison of Arctic and Alpine plant function under the snowpack. Arct Antarct Alp Res 32:404–411. doi:10.2307/1552389
Bintanja R, Selten FM (2014) Future increases in Arctic precipitation linked to local evaporation and sea–ice retreat. Nature 509:479–482
Blok D, Sass-Klaassen U, Schaepman-Strub G, Heijmans MMPD, Sauren P, Berendse F (2011a) What are the main climate drivers for shrub growth in Northeastern Siberian tundra? Biogeosciences 8:1169–1179. doi:10.5194/bg-8-1169-2011
Blok D, Schaepman-Strub G, Bartholomeus H, Heijmans MMPD, Maximov TC, Berendse F (2011b) The response of Arctic vegetation to the summer climate: relation between shrub cover, NDVI, surface albedo and temperature. Environ Res Lett 6. doi:10.1088/1748-9326/6/3/035502
Blok D, Weijers S, Welker JM, Cooper EJ, Michelsen A, Löffler J, Elberling B (2015) Deepened winter snow increases stem growth and alters stem δ 13 C and δ 15 N in evergreen dwarf shrub Cassiope tetragona in high-arctic Svalbard tundra. Environ Res Lett 10. doi:10.1088/1748-9326/10/4/044008
Bradley RS, Jones PD (1992) Climate since A.D. 1500. Routledge, London
Bret-Harte MS et al (2008) Plant functional types do not predict biomass responses to removal and fertilization in Alaskan tussock tundra. J Ecol 96:713–726. doi:10.1111/j.1365-2745.2008.01378.x
Briffa KR et al (2008) Trends in recent temperature and radial tree growth spanning 2000 years across northwest Eurasia. Philos Trans Biol Sci 363:2271–2284. doi:10.2307/20208637
Buchwal A, Rachlewicz G, Fonti P, Cherubini P, Gärtner H (2013) Temperature modulates intra-plant growth of Salix polaris from a high Arctic site (Svalbard). Polar Biol 36:1305–1318. doi:10.1007/s00300-013-1349-x
Bunn AG (2008) A dendrochronology program library in R (dplR). Dendrochronologia 26:115–124. doi:10.1016/j.dendro.2008.01.002
Chapin FS III (1980) Nutrient allocation and responses to defoliation in tundra plants. Arct Alp Res 12:553–563. doi:10.2307/1550500
Chapin FS III, Schulze E-D, Mooney HA (1990) The ecology and economics of storage in plants. Annu Rev Ecol Syst 21:423–447. doi:10.2307/2097032
Cook ER, Holmes RL (1986) Users manual for program ARSTAN. University of Arizona, Laboratory of tree-ring research, Tucson
Frost GV, Epstein HE (2014) Tall shrub and tree expansion in Siberian tundra ecotones since the 1960s. Glob Change Biol 20:1264–1277. doi:10.1111/gcb.12406
Gärtner H, Lucchinetti S, Schweingruber FH (2014) New perspectives for wood anatomical analysis in dendrosciences: the GSL1-microtome. Dendrochronologia 32:47–51. doi:10.1016/j.dendro.2013.07.002
Groot WJd, Thomas PA, Wein RW (1997) Betula nana L. and Betula glandulosa Michx. J Ecol 85:241–264. doi:10.2307/2960655
Hallinger M, Manthey M, Wilmking M (2010) Establishing a missing link: warm summers and winter snow cover promote shrub expansion into alpine tundra in Scandinavia. N Phytol 186:890–899. doi:10.1111/j.1469-8137.2010.03223.x
Hinzman L et al (2005) Evidence and implications of recent climate change in northern Alaska and other Arctic regions. Clim Change 72:251–298. doi:10.1007/s10584-005-5352-2
Hobbie SE, Chapin FS III (1998) The response of tundra plant biomass, aboveground production, nitrogen, and CO2 flux to experimental warming. Ecology 79:1526–1544. doi:10.2307/176774
Hollesen J, Buchwal A, Rachlewicz G, Hansen BU, Hansen MO, Stecher O, Elberling B (2015) Winter warming as an important co-driver for Betula nana growth in western Greenland during the past century. Glob Change Biol 21:2410–2423. doi:10.1111/gcb.12913
Hudson JMG, Henry GHR (2009) Increased plant biomass in a High Arctic heath community from 1981 to 2008. Ecology 90:2657–2663. doi:10.1890/09-0102.1
IPCC (2013) Climate change 2013: the physical science basis. Cambridge University Press, Cambridge
Jonasson S (1982) Organic matter and phytomass on three north Swedish tundra sites, and some connections with adjacent tundra areas. Ecography 5:367–375. doi:10.1111/j.1600-0587.1982.tb01050.x
Juszak I, Erb AM, Maximov TC, Schaepman-Strub G (2014) Arctic shrub effects on NDVI, summer albedo and soil shading. Remote Sens Environ 153:79–89. doi:10.1016/j.rse.2014.07.021
Kolishchuk V (1990) Dendroclimatological study of prostrate woody plants. In: Cook E, Kairiukstis L (eds) Methods of dendrochronology applications in the environmental sciences. Kluwer, London, pp 51–55
Liang M et al (2014) Importance of soil moisture and N availability to larch growth and distribution in the Arctic taiga-tundra boundary ecosystem, northeastern Siberia. Polar Sci 8:327–341. doi:10.1016/j.polar.2014.07.008
Lloyd AH, Yoshikawa K, Fastie CL, Hinzman L, Fraver M (2003) Effects of permafrost degradation on woody vegetation at arctic treeline on the Seward Peninsula, Alaska. Permafr Periglac Process 14:93–101. doi:10.1002/ppp.446
Loranty M, Goetz S, Rastetter E, Rocha A, Shaver G, Humphreys E, Lafleur P (2011) Scaling an instantaneous model of tundra NEE to the Arctic landscape. Ecosystems 14:76–93. doi:10.1007/s10021-010-9396-4
Mack MC, Schuur EAG, Bret-Harte MS, Shaver GR, Chapin FS (2004) Ecosystem carbon storage in Arctic tundra reduced by long-term nutrient fertilization. Nature 431:440–443
Mäkinen H et al (2003) Large-scale climatic variability and radial increment variation of Picea abies (L.) Karst. in central and northern Europe. Trees 17:173–184. doi:10.1007/s00468-002-0220-4
Marschner P, Rengel Z, Stark S (2007) Nutrient cycling in the tundra. In: Marschner P, Rengel Z (eds) Nutrient cycling in terrestrial ecosystems. Springer, Berlin, pp 309–331
McGuire AD, Chapin FS, Walsh JE, Wirth C (2006) Integrated regional changes in Arctic climate feedbacks: implications for the global climate system. Annu Rev Environ Resour 31:61–91. doi:10.1146/annurev.energy.31.020105.100253
Meinardus C, Weinert B, Löffler J, Lundberg A, Bräuning A (2011) The potential of the dwarf shrub Betula nana L. as a climate indicator above the tree line in the southern Norwegian Scandes. Potsdam
Miller H (1975) Anatomical characteristics of some woody plants of the Angmagssalik district of Southeast Greenland. Medd Gronland 198:1–30
Myers-Smith IH et al (2011) Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environ Res Lett. doi:10.1088/1748-9326/6/4/045509
Myers-Smith IH et al (2015a) Climate sensitivity of shrub growth across the tundra biome. Nat Clim Change 5:887–891
Myers-Smith IH et al (2015b) Methods for measuring Arctic and Alpine shrub growth: a review. Earth Sci Rev 140:1–13
Myneni RB, Keeling CD, Tucker CJ, Asrar G, Nemani RR (1997) Increased plant growth in the northern high latitudes from 1981 to 1991. Nature 386:698–702
Nauta AL et al (2015) Permafrost collapse after shrub removal shifts tundra ecosystem to a methane source. Nat Clim Change 5:67–70. doi:10.1038/nclimate2446
Nowinski N, Taneva L, Trumbore S, Welker J (2010) Decomposition of old organic matter as a result of deeper active layers in a snow depth manipulation experiment. Oecologia 163:785–792. doi:10.1007/s00442-009-1556-x
Parmentier FJW et al (2011) Longer growing seasons do not increase net carbon uptake in Northeastern Siberian tundra. Biogeosciences 116:1091–1095. doi:10.1029/2011JG001653
Pop EW, Oberbauer SF, Starr G (2000) Predicting vegetative bud break in two Arctic deciduous shrub species, Salix pulchra and Betula nana. Oecologia 124:176–184. doi:10.1007/s004420050005
Schweingruber FH (1996) Tree rings and environment dendroecology. Paul Haupt Publishers, Berne
Schweingruber FH, Borner A, Schulze ED (2011) Altas of stem anatomy in herbs, shrubs and trees. Springer, Berlin
Schweingruber FH, Hellmann L, Tegel W, Braun S, Nievergelt D, Büntgen U (2013) Evaluating the wood anatomical and dendroecological potential of arctic dwarf shrub communities. Iawa J 34:485–497
Shaver GR, Bret-Harte MS, Jones MH, Johnstone J, Gough L, Laundre J, Chapin FS III (2001) Species composition interacts with fertilizer to control long-term change in tundra productivity. Ecology 82:3163–3181
Stow DA et al (2004) Remote sensing of vegetation and land-cover change in Arctic tundra ecosystems. Remote Sens Environ 89:281–308. doi:10.1016/j.rse.2003.10.018
Sturm M, Holmgren J, McFadden JP, Liston GE, Chapin FS III, Racine CH (2001) Snow–shrub interactions in Arctic tundra: a hypothesis with climatic implications. J Clim 14:336–344. doi:10.1175/1520-0442(2001)014
Sturm M, Douglas T, Racine C, Liston GE (2005) Changing snow and shrub conditions affect albedo with global implications. Biogeosciences 110:195–221. doi:10.1029/2005JG000013
Tape KEN, Sturm M, Racine C (2006) The evidence for shrub expansion in Northern Alaska and the Pan-Arctic. Glob Change Biol 12:686–702. doi:10.1111/j.1365-2486.2006.01128.x
Urban M, Forkel M, Eberle J, Hüttich C, Schmullius C, Herold M (2014) Pan-Arctic climate and land cover trends derived from multi-variate and multi-scale analyses (1981–2012). Remote Sens 6:2296
Vicente-Serrano SM, Beguería S, López-Moreno JI (2010) A multiscalar drought index sensitive to global warming: the standardized precipitation evapotranspiration index. J Clim 23:1696–1718
Wahren CHA, Walker MD, Bret-Harte MS (2005) Vegetation responses in Alaskan Arctic tundra after 8 years of a summer warming and winter snow manipulation experiment. Glob Change Biol 11:537–552. doi:10.1111/j.1365-2486.2005.00927.x
Walker DA, Billings WD, De Molenaar JG (1997) Snow–vegetation interactions in tundra environments. In: Jones HG, Pomeroy JW, Walker DA, Hoham RW (eds) Snow ecology: an interdisciplinary examination of snow-covered ecosystems. Cambridge University Press, New York, pp 266–324
Walker DA et al (2005) The circumpolar Arctic vegetation map. J Veg Sci 16:267–282. doi:10.1111/j.1654-1103.2005.tb02365.x
Walker MD et al (2006) Plant community responses to experimental warming across the tundra biome. Proc Nat Acad Sci USA 103:1342–1346. doi:10.1073/pnas.0503198103
Wilmking M et al (2012) Continuously missing outer rings in woody plants at their distributional margins. Dendrochronologia 30:213–222. doi:10.1016/j.dendro.2011.10.001
Woodcock H, Bradley RS (1994) Salix arctica (Pall): its potential for dendroclimatological studies in the High Arctic. Dendrochronologia 12:11–22
Acknowledgments
This study is financed by the Darwin Center for Biogeosciences, Wageningen Institute for Environment and Climate Research (WIMEK) and the Netherlands Organization for Scientific Research (NWO, Vidi Grant 864.09.014). Daan Blok was funded through a grant by the Danish National Research Foundation (CENPERM DNRF100). We kindly thank Stas Ksenofontov and other staff of the IBPC institute, Yakutsk, and staff of the Regional Inspection of Nature Protection of Allaikhovsky Region, Chokurdakh, for logistic support. We thank Jelmer Nijp, Natali Oram, Joost Huttenga and Marjolein Mann for their helpful comments on the manuscript. This study was inspired by discussion within the framework of the COST Action FP1106, STReESS.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Li, B., Heijmans, M.M.P.D., Berendse, F. et al. The role of summer precipitation and summer temperature in establishment and growth of dwarf shrub Betula nana in northeast Siberian tundra. Polar Biol 39, 1245–1255 (2016). https://doi.org/10.1007/s00300-015-1847-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1847-0