Abstract
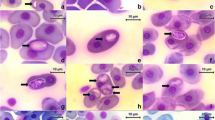
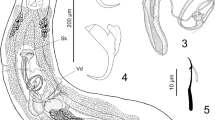
Antarctic krill are parasitized by gregarines (Phylum Apicomplexa, Class Sporozoea, Order Eugregarinida), which were observed in this study by light, scanning, and transmission electron microscopy. Eighty seven percentage of the krill examined (n = 93) were infected with the parasites in the cephalin stage, which were regularly found in the hind-gut epithelium of their host. Cephalins were found attached to the epithelium, and then were liberated into the intestinal lumen. The gamont stage, which follows the cephalin stage, was found in the intestinal lumen, as well as the diverticulum of mid-gut gland. However, gamonts found in the mid-gut gland were considerably larger and elongated in comparison with those in the intestinal lumen. Gamonts in the diverticulum appear to damage microvilli, which are involved in the uptake of digested nutrients and secretion of various enzymes. Therefore, elevated infestation of this parasite in the mid-gut gland may have a significant impact on the nutritional state of the Antarctic krill host.





Similar content being viewed by others
References
Avdeev VV (1985) New species of gregarines of genus Cephaloidophora: parasites of Euphausia superba. Parasitologiya 19:72–75
Avdeev VV (1987) Certain specific characteristics of the development of gregarina Cephaloidophora pacifica, a parasite of Euphausia superba. Parasitologiya 21:580–582
Avdeev VV, Vagin AV (1987) On the pathogenic effect of Cephaloidophora pacifica Avdeev on Euphausia superba Dana. Parasitologiya 21:741–743
Begon M, Harper JL, Townsend CD (1996) Ecology: individuals, populations and communities, 3rd edn. Blackwell
Cadee GC, Gonzalez H, Schnack-Schiel SB (1992) Krill diet affects fecal string setting. Polar Biol 12:75–80
Clopton R (1995) Leidyana migratory n. sp. (Apicomplexa: Eugregarinidia: Leidyanidae) form the Madagascar hissing cockroach, Gromphadorhina portentosa (Insecta: Blattodea). Invertebr Biol 114:271–278
Clopton RE, Gold RE (1996) Host specificity of Gregarina blattarum von Siebold, 1839 (Apicomplexa: Eugregarinida) among five species of domiciliary cockroaches. J Invertebr Pathol 67:219–223
Clopton RE, Percival TJ, Janovy J Jr (1992) Gregarina coronata n. sp. (Apicomplexa: Eugregarinida) described from adults of the southern corn rootworm, Diabrotica undecimpunctata howardi (Coleoptera: Chrysomelidae). J Protozool 39:417–420
Dall W, Moriarly DJW (1983) Functional aspects of nutrition and digestion. In: Mantal LH (ed) The biology of Crustacea, vol 5. Academic Press, New York, pp 215–261
Farmer JN (1980) The protozoa: Introduction to protozoology. CV Mosby Co., St. Louis, Toronto, London, 732 p
Hamner WM, Hamner PP, Strand SW, Gilmer RW (1983) Behavior of Antarctic krill, Euphausia superba: chemoreception, feeding, schooling and molting. Science 220:433–435
Hempel G (1985) Antarctic marine food web. In: Siegfried WR, Condy PR, Laws RM (eds) Antarctic nutrient cycles and food webs. Springer, Berlin, pp 266–270
Hoshide K (1969) Studies on gregarines from Japan I. Cepahloidophora warekara n. sp. and two other gregarines from crustaceans. J Fac Sci Hokkaido Univ Ser VI Zool 17:6–16
Hoshide K (1993) A new species of septate gregarine (Protozoa; Gregarinasina) from a marine amphipoda Ampithoe lacertosa Bate. Proc Jap Soc syst Zool 48:1–6
Hoshide K, Todd KS (1993) The fine structure of cell surface and hair-like projections of Filipodium ozaki Hukui 1939 gamonts. Acta Protozool 35:309–315
Hoshide K, Nakamura H, Todd KS (1993) In vitro excyxtation of Gregarina blattarum oocysts. Acta Protozool 32:67–70
Ikeda T, Nash GV, Thomas PG (1984) An observation of discarded stomach with exoskeleton moult from Antarctic krill Euphausia superba Dana. Polar Biol 3:241–244
Kagei N, Asano K, Kihata M (1978) On the examination against the parasites of Antarctic krill, Euphausia superba. Sci Repts Whales Res Inst Tokyo 30:311–313
Kamm MW (1922) Studies on gregarines II. Synopsis of the polycystid gregarines of the world, excluding those from the Myriapoda, Orthoptera and Coleoptera. Illin BIol Monog 7, pp 104
Kawaguchi S, Toda T (1997) Discovery of ciliates reproducing in the gut of Antarctic krill. Polar Biol 18:158–160
Kawaguchi S, Candy S, King R, Naganobu M, Nicol S (2006) Modelling growth of Antarctic krill. I. Growth trends with sex, length season, and region. Mar Ecol Prog Ser 306:1–15
Levine ND (1971) Uniform terminology for the protozoan subphylum apicomplexa. J Protozool 18:352–355
Levine ND (1988) The protozoan phylum Apicomplexa. Chemical Rubber Company Press, Boca Raton, Florida 2 Vols
Mackintosh NA (1964) A survey of Antarctic biology up to 1945. In: Carrick R, Holdgate MW, Prevost J (eds) Biologie antarctique. Hermann, Paris, pp 3–38
Marr JWS (1962) The natural history and geography of the Antarctic krill (Euphausia superba Dana). Discov Rep 32:33–464
Mauchline J (1980) The biology of mysids and eupahusiids. In: Blaxer JHS, Russel FS, Yonge M (eds) Advances in marine biology, vol 18. Academic Press, London, pp 1–681
Nicol S, Kitchener J, King R, Hosie G, de la Mare WK (2000) Population structure and condition of Antarctic krill (Euphausia superba) off east Antarctica (80–150°E) during the Austral summer of 1995/1996. Deep Sea Res II 47:2489–2517
Rakusa-Suszczewski S, Filcek K (1988) Protozoa on the body on Euphausia superba Dana from Admiralty Bay (the South Shetland Islands). Acta Protozool 27:21–30
Rakusa-Suszczewski S, Nemoto T (1989) Ciliates associations on the body of krill (Euphausia superba Dana). Acta Protozool 28:77–86
Takahashi KT, Kawaguchi S, Kobayashi M, Toda T (2003) Parasitic eugregarines change their spatial distribution within the host digestive tract of Antarctic krill, Euphausia superba. Polar Biol 26:468–473
Takahashi KT, Kawaguchi S, Kobayashi M, Toda T (2004) The variability in abundance of eugregarines living in the Antarctic krill. Polar Biosci 17:16–25
Takahashi KT, Kobayashi M, Kawaguchi S, Saigusa J, Tanimura A, Fukuchi M, Naganobu M, Toda T (2008) Circumpolar occurrence of eugregarinid protozoan Cephaloidophora pacifica associated with Antarctic krill, Euphausia superba. Ant Sci 20:437–440
Théodoridès J (1989) Parasitology of marine zooplankton. Adv Mar Biol 25:117–177
Walker MH, Mackenzie C, Bainbridge SP, Orme C (1979) A study of the structure and gliding movement of Gregarina garnhami. J Protozool 26:566–574
Zuk M (1987) The effects of gregarine parasites on longevity, weight loss, fecundity and developmental time in the field crickets Gryllus veletis and G. pennsylvanicus. Ecol Entomol 12:349–354
Acknowledgments
We thank the officers and crew of the RSV Aurora Australis, Australian National Antarctic Research Expeditions for their kind assistance during the cruise. We are grateful to Drs. Graham W. Hosie, Stephen Nicol, Anna McEldowney, Gerry Nash, and Harvey J. Marchant, Australian Antarctic Division, Mikio Naganobu and Taro Ichii, National Research Institute of Far Seas Fisheries, Mr. Masaki Kobayashi, Soka University, for various advises and supports throughout this study. The Japan Society for the Promotion of Science also is acknowledged for financial support during this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takahashi, K.T., Kawaguchi, S. & Toda, T. Observation by electron microscopy of a gregarine parasite of Antarctic krill: its histological aspects and ecological explanations. Polar Biol 32, 637–644 (2009). https://doi.org/10.1007/s00300-008-0563-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-008-0563-4