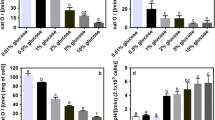
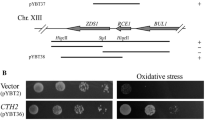
Abstract
When glucose is available, Saccharomyces cerevisiae prefers fermentation to respiration. In fact, it can live without respiration at all. Here, we study the role of respiration in stress tolerance in yeast. We found that colony growth of respiratory-deficient yeast (petite) is greatly inhibited by canavanine, the toxic analog of arginine that causes proteotoxic stress. We found lower amounts of the amino acids involved in arginine biosynthesis in petites compared with WT. This finding may be explained by the fact that petite cells exposed to canavanine show reduction in the efficiency of targeting of proteins required for arginine biosynthesis. The retrograde (RTG) pathway signals mitochondrial stress. It positively controls production of arginine precursors. We show that canavanine abrogates RTG signaling especially in petite cells, and mutants in the RTG pathway are extremely sensitive to canavanine. We suggest that petite cells are naturally ineffective in production of some amino acids; combination of this fact with the effect of canavanine on the RTG pathway is the simplest explanation why petite cells are inhibited by canavanine. Surprisingly, we found that canavanine greatly inhibits colony formation when WT cells are forced to respire. Our research proposes a novel connection between respiration and proteotoxic stress.













Similar content being viewed by others
References
Ahmad M, Bussey H (1986) Yeast arginine permease: nucleotide sequence of the CAN1 gene. Curr Genet 10(8):587–592
Anderson Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PHJ, Smith AJ, Staden R, Young IG (1981) Sequence and organization of the human mitochondrial genome. Nature 290(5806):457–465
Becker-Kettern J, Paczia N, Conrotte J-F, Kay DP, Guignard C, Jung PP, Linster CL (2016) Saccharomyces cerevisiae forms d-2-hydroxyglutarate and couples its degradation to d-lactate formation via a cytosolic transhydrogenase. J Biol Chem 291(12):6036–6058. https://doi.org/10.1074/jbc.m115.704494 (Epub 2016 Jan 16)
Burns N, Grimwade B, Ross-Macdonald PB, Choi EY, Finberg K, Roeder GS, Snyder M (1994) Large-scale analysis of gene expression, protein localization, and gene disruption in Saccharomyces cerevisiae. Genes Dev 8(9):1087–1105
Camougrand N, Kissová I, Salin B, Devenish RJ (2008) Chapter 8 monitoring mitophagy in yeast. Methods in enzymology. Academic, New York, pp 89–107
Carlson M (1999) Glucose repression in yeast. Curr Opin Microbiol 2(2):202–207
Chelstowska A, Butow RA (1995) RTG genes in yeast that function in communication between mitochondria and the nucleus are also required for expression of genes encoding peroxisomal proteins. J Biol Chem 270(30):18141–18146
Chelstowska A, Liu Z, Jia Y, Amberg D, Butow RA (1999) Signalling between mitochondria and the nucleus regulates the expression of a new d-lactate dehydrogenase activity in yeast. Yeast 15(13):1377–1391
Chen XJ, Clark-Walker GD (2000) The petite mutation in yeasts: 50 years on. Int Rev Cytol 194:197–238
Contamine V, Picard M (2000) Maintenance and integrity of the mitochondrial genome: a plethora of nuclear genes in the budding yeast. Microbiol Mol Biol Rev 64(2):281–315
Contreras-Shannon V, Lin AP, McCammon MT, McAlister-Henn L (2005) Kinetic properties and metabolic contributions of yeast mitochondrial and cytosolic NADP + -specific isocitrate dehydrogenases. J Biol Chem 280(6):4469–4475
Day M (2013) Yeast petites and small colony variants: for everything there is a season. Adv Appl Microbiol 85:1–41
de Alteriis E, Carteni F, Parascandola P, Serpa J, Mazzoleni S (2018) Revisiting the Crabtree/Warburg effect in a dynamic perspective: a fitness advantage against sugar-induced cell death. Cell Cycle 17(6):688–701. https://doi.org/10.1080/15384101.2018.1442622
de Deken RH (1966) The Crabtree effect: a regulatory system in yeast. J Gen Microbiol 44(2):149–156. https://doi.org/10.1099/00221287-44-2-149
DeLuna A, Avendano A, Riego L, Gonzalez A (2001) A NADP-glutamate dehydrogenase isoenzymes of Saccharomyces cerevisiae. Purification, kinetic properties, and physiological roles. J Biol Chem 276(47):43775–43783
Epstein CB, Waddle JA, Hale W, Davé V, Thornton J, Macatee TL, Garner HR, Butow RA (2001) Genome-wide responses to mitochondrial dysfunction. Mol Biol Cell 12(2):297–308
Evans A, Neuman N (2016) The mighty mitochondria. Mol Cell 61(5):641. https://doi.org/10.1016/j.molcel.2016.02.002
Forsburg SL, Guarente L (1989) Communication between mitochondria and the nucleus in regulation of cytochrome genes in the yeast Saccharomyces cerevisiae. Annu Rev Cell Biol 5:153–180
Gancedo JM (1998) Yeast carbon catabolite repression. Microbiol Mol Biol Rev 62(2):334–361
Giaever G, Chu AM, Ni L, Connelly C, Riles L, Veronneau S, Dow S, Lucau-Danila A, Anderson K, Andre B, Arkin AP, Astromoff A, El-Bakkoury M, Bangham R, Benito R, Brachat S, Campanaro S, Curtiss M, Davis K, Deutschbauer A, Entian KD, Flaherty P, Foury F, Garfinkel DJ, Gerstein M, Gotte D, Güldener U, Hegemann JH, Hempel S, Herman Z et al (2002) Functional profiling of the Saccharomyces cerevisiae genome. Nature 418(6896):387–391
Goldring ES, Grossman LI, Krupnick D, Cryer DR, Marmur J (1970) The petite mutation in Yeast: loss of mitochondrial deoxyribonucleic acid during induction of petites with ethidium bromide. J Mol Biol 52:323–335. https://doi.org/10.1016/0022-2836(70)90033-1
Guaragnella N, Butow RA (2003) ATO3 encoding a putative outward ammonium transporter is an RTG-independent retrograde responsive gene regulated by GCN4 and the Ssy1-Ptr3-Ssy5 amino acid sensor system. J Biol Chem 278(46):45882–45887
Guillamon JM, van Riel NA, Giuseppin ML, Verrips CT (2001) The glutamate synthase (GOGAT) of Saccharomyces cerevisiae plays an important role in central nitrogen metabolism. FEMS Yeast Res 1(3):169–175
Honlinger A, Kubrich M, Moczko M, Gartner F, Mallet L, Bussereau F, Eckerskorn C, Lottspeich F, Dietmeier K, Jacquet M et al (1995) The mitochondrial receptor complex: Mom22 is essential for cell viability and directly interacts with preproteins. Mol Cell Biol 15(6):3382–3389
Huh WK, Falvo JV, Gerke LC, Gerke LC, Carroll AS, Howson RW, Weissman JS, O’Shea EK (2003) Global analysis of protein localization in budding yeast. Nature 425(6959):686–691
Jambhekar A, Amon A (2008) Control of meiosis by respiration. Curr Biol 18(13):969–975. https://doi.org/10.1016/j.cub.2008.05.047
Jauniaux JC, Urrestarazu LA, Wiame JM (1978) Arginine metabolism in Saccharomyces cerevisiae: subcellular localization of the enzymes. J Bacteriol 133(3):1096–1107
Kanki T, Kang D, Klionsky DJ (2009) Monitoring mitophagy in yeast: the Om45-GFP processing assay. Autophagy 5(8):1186–1189
Knupp J, Arvan P, Chang A (2019) Increased mitochondrial respiration promotes survival from endoplasmic reticulum stress. Cell Death Differ 26(3):487–501. https://doi.org/10.1038/s41418-018-0133-4 (Epub 2018 May 23)
Komeili A, Wedaman KP, O’Shea EK, Powers T (2000) Mechanism of metabolic control. Target of rapamycin signaling links nitrogen quality to the activity of the Rtg1 and Rtg3 transcription factors. J Cell Biol 151(4):863–878
Larosa V, Remacle C (2018) Insights into the respiratory chain and oxidative stress. Biosci Rep 38(5):BSR20171492
Lewin AS, Hines V, Small GM (1990) Citrate synthase encoded by the CIT2 gene of Saccharomyces cerevisiae is peroxisomal. Mol Cell Biol 10(4):1399–1405
Liao X, Butow RA (1993) RTG1 and RTG2: Two yeast genes required for a novel path of communication from mitochondria to the nucleus. Cell 72(1):61–71
Liao XS, Small WC, Srere PA, Butow RA (1991) Intramitochondrial functions regulate nonmitochondrial citrate synthase (CIT2) expression in Saccharomyces cerevisiae. Mol Cell Biol 11(1):38–46
Lithgow T, Junne T, Suda K, Gratzer S, Schatz G (1994) The mitochondrial outer membrane protein Mas22p is essential for protein import and viability of yeast. Proc Natl Acad Sci USA 91(25):11973–11977
Liu Z, Butow RA (1999) A transcriptional switch in the expression of yeast tricarboxylic acid cycle genes in response to a reduction or loss of respiratory function. Mol Cell Biol 19(10):6720–6728
Liu Z, Butow RA (2006) Mitochondrial retrograde signaling. Annu Rev Genet 40:159–185
Liu Z, Sekito T, Spirek M, Thornton J, Butow RA (2003) Retrograde signaling is regulated by the dynamic interaction between Rtg2p and Mks1p. Mol Cell 12(2):401–411
Magasanik B, Kaiser CA (2002) Nitrogen regulation in Saccharomyces cerevisiae. Gene 290(1–2):1–18
Martiez-Force E, Benitez T (1992) Changes in yeast amino acid pool with respiratory versus fermentative metabolism. Biotechnol Bioeng 40(6):643–649
Merico A, Sulo P, Piškur J, Compagno C (2007) Fermentative lifestyle in yeasts belonging to the Saccharomyces complex. FEBS J 274(4):976–989
Moye-Rowley WS (2005) Retrograde regulation of multidrug resistance in Saccharomyces cerevisiae. Gene 354:15–21
Myung K, Chen C, Kolodner RD (2001) Multiple pathways cooperate in the suppression of genome instability in Saccharomyces cerevisiae. Nature 411(6841):1073–1076
Peng Y, Dong D, Jiang C, Yu B, Wang X, Ji Y (2012) Relationship between respiration deficiency and azole resistance in clinical Candida glabrata. FEMS Yeast Res 12(6):719–727. https://doi.org/10.1111/j.1567-1364.2012.00821.x (Epub 2012 Jul 11)
Prevost CT, Peris N, Seger C, Pedeville DR, Wershing K, Sia EA, Sia RAL (2018) The influence of mitochondrial dynamics on mitochondrial genome stability. Curr Genet 64(1):199–214. https://doi.org/10.1007/s00294-017-0717-4 (Epub 2017 Jun 1)
Quezada H, Marin-Hernandez A, Arreguin-Espinosa R, Rumjanek FD, Moreno-Sanchez R, Saavedra E (2013) The 2-oxoglutarate supply exerts significant control on the lysine synthesis flux in Saccharomyces cerevisiae. FEBS J 280(22):5737–5749
Rodrigues F, Ludovico P, Leão C (2006) Sugar metabolism in yeasts: an overview of aerobic and anaerobic glucose catabolism. Biodiversity and ecophysiology of yeasts. Springer, Berlin, pp 101–121
Roon RJ, Even HL, Larimore F (1974) Glutamate synthase: properties of the reduced nicotinamide adenine dinucleotide-dependent enzyme from Saccharomyces cerevisiae. J Bacteriol 118(1):89–95
Rosenthal GA (1977) The biological effects and mode of action of L-canavanine, a structural analogue of l-arginine. Q Rev Biol 52(2):155–178
Scalliet G, Bowler J, Luksch T, Kirchhofer-Allan L, Steinhauer D, Ward K, Niklaus M, Verras A, Csukai M, Daina A, Fonné-Pfister R (2012) Mutagenesis and functional studies with succinate dehydrogenase inhibitors in the wheat pathogen Mycosphaerella graminicola. PLoS One 7(4):e35429
Sesaki H, Southard SM, Yaffe MP, Jensen RE (2003) Mgm1p, a dynamin-related GTPase, is essential for fusion of the mitochondrial outer membrane. Mol Biol Cell 14(6):2342–2356 (Epub 2003 Feb 6)
Shi Y, Stefan CJ, Rue SM, Teis D, Emr SD (2011) Two novel WD40 domain-containing proteins, Ere1 and Ere2, function in the retromer-mediated endosomal recycling pathway. Mol Biol Cell 22(21):4093–4107
Shor E, Fox CA, Broach JR (2013) The yeast environmental stress response regulates mutagenesis induced by proteotoxic stress. PLoS Genet 9:e1003680. https://doi.org/10.1371/journal.pgen.1003680
Simpson-Lavy K, Xu T, Johnston M, Kupiec M (2017) The Std1 activator of the Snf1/AMPK kinase controls glucose response in yeast by a regulated protein aggregation. Mol Cell 68:1120–1133. https://doi.org/10.1016/j.molcel.2017.11.016
Smith EH, Janknecht R, Maher LJ III (2007) Succinate inhibition of α-ketoglutarate-dependent enzymes in a yeast model of paraganglioma. Hum Mol Genet 16(24):3136–3148
Soontorngun N (2017) Reprogramming of nonfermentative metabolism by stress-responsive transcription factors in the yeast Saccharomyces cerevisiae. Curr Genet 63(1):1–7. https://doi.org/10.1007/s00294-016-0609-z (Epub 2016 May 14)
Storici F, Resnick MA (2006) The delitto perfetto approach to in vivo site-directed mutagenesis and chromosome rearrangements with synthetic oligonucleotides in yeast. Methods Enzymol 409:329–345
Torelli NQ, Ferreira-Júnior JR, Kowaltowski AJ, da Cunha FM (2015) RTG1- and RTG2-dependent retrograde signaling controls mitochondrial activity and stress resistance in Saccharomyces cerevisiae. Free Radic Biol Med 81:30–37
Traven A, Wong JM, Xu D, Sopta M, Ingles CJ (2001) Interorganellar communication Altered nuclear gene expression profiles in a yeast mitochondrial DNA mutant. J Biol Chem 276(6):4020–4027
Vlahakis A, Lopez Muniozguren N, Powers T (2017) Mitochondrial respiration links TOR complex 2 signaling to calcium regulation and autophagy. Autophagy 13(7):1256–1257. https://doi.org/10.1080/15548627.2017.1299314 (Epub 2017 Mar 21)
Williamson DH, Maroudas NG, Wilkie D (1971) Induction of the cytoplasmic petite mutation in Saccharomyces cerevisiae by the antibacterial antibiotics erythromycin and chloramphenicol. Mol Gen Genet 111(3):209–223
Wodicka L, Dong H, Mittmann M, Ho M-H, Lockhart DJ (1997) Genome-wide expression monitoring in Saccharomyces cerevisiae. Nat Biotechnol 15(13):1359–1367. https://doi.org/10.1038/nbt1297-1359
Woelders H (1989) Mitochondrial oxidative phosphorylation: studies on the chemiosmotic coupling between respiration and ATP synthesis. Dissertation, University of Amsterdam
Zelenaya-Troitskaya O, Perlman PS, Butow RA (1995) An enzyme in yeast mitochondria that catalyzes a step in branched-chain amino acid biosynthesis also functions in mitochondrial DNA stability. EMBO J 14(13):3268–3276
Zhang N, Cao L (2017) Starvation signals in yeast are integrated to coordinate metabolic reprogramming and stress response to ensure longevity. Curr Genet 63(5):839–843. https://doi.org/10.1007/s00294-017-0697-4 (Epub 2017 Apr 25)
Zhang H, Singh KK (2014) Global genetic determinants of mitochondrial DNA copy number. PLoS One 9(8):e105242
Acknowledgements
We thank the Schuldiner lab for technical support and most valuable input. This work was supported by RCDA Grant of ICRF to SC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Arginine biosynthesis pathway.
Original representation based on arginine biosynthesis pathway from SGD database (yeastgenome.org). Red denotes location in mitochondria; blue denotes location in cytosol (TIFF 1540 kb)
294_2019_974_MOESM2_ESM.tiff
can1Δis sensitive to growth on a non-fermentable carbon source with canavanine. A can1Δ mutant was pronged onto glucose (-) arginine with 20 μg/ml canavanine and lactic acid (-) arginine with 20 μg/ml canavanine. Growth after one week (TIFF 104 kb)
Arginine addition somewhat rescues petites.
Strains were grown on 2 µg/ml canavanine glucose (-) arginine and glucose with increasing amounts of arginine. Growth after 10 days (TIFF 739 kb)
Rights and permissions
About this article
Cite this article
Druseikis, M., Ben-Ari, J. & Covo, S. The Goldilocks effect of respiration on canavanine tolerance in Saccharomyces cerevisiae. Curr Genet 65, 1199–1215 (2019). https://doi.org/10.1007/s00294-019-00974-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-019-00974-y