Abstract
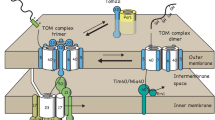
In this report, we summarize recent findings about a role of the outer membrane metabolite channel VDAC/porin in protein import into mitochondria. Mitochondria fulfill key functions for cellular energy metabolism. Their biogenesis involves the import of about 1000 different proteins that are produced as precursors on cytosolic ribosomes. The translocase of the outer membrane (TOM complex) forms the entry gate for mitochondrial precursor proteins. Dedicated protein translocases sort the preproteins into the different mitochondrial subcompartments. While protein transport pathways are analyzed to some detail, only little is known about regulatory mechanisms that fine-tune protein import upon metabolic signaling. Recently, a dual role of the voltage-dependent anion channel (VDAC), also termed porin, in mitochondrial protein biogenesis was reported. First, VDAC/porin promotes as a coupling factor import of carrier proteins into the inner membrane. Second, VDAC/porin regulates the formation of the TOM complex. Thus, the major metabolite channel in the outer membrane VDAC/porin connects protein import to mitochondrial metabolism.

Similar content being viewed by others
References
Alconada A, Kübrich M, Moczko M, Hönlinger A, Pfanner N (1995) The mitochondrial receptor complex: the small subunit Mom8b/Isp6 supports association of receptors with the general insertion pore and transfer preproteins. Mol Cell Biol 15:6196–6205
Bausewein T, Mills DJ, Langer JD, Nitschke B, Nussberger S, Kühlbrandt W (2017) Cryo-EM structure of the TOM core complex from Neurospora crassa. Cell 170:693–700
Becker T, Wenz LS, Thornton N, Stroud DA, Meisinger C, Wiedemann N, Pfanner N (2011) Biogenesis of mitochondria: dual role of Tom7 in modulating assembly of the preprotein translocase of the outer membrane. J Mol Biol 405:113–124
Becker T, Wagner R (2018) Mitochondrial outer membrane channels: emerging diversity in transport processes. BioEssays 40(7):1800013
Böttinger L, Mårtensson CU, Song J, Zufall N, Wiedemann N, Becker T (2018) Respiratory chain supercomplexes associate with the cysteine desulfurase complex of the iron-sulfur cluster assembly machinery. Mol Biol Cell 29:776–785
Campo ML, Peixoto PM, Martinez-Caballero S (2017) Revisiting trends on mitochondrial mega-channels for the import of proteins and nucleic acids. J Bioenerg Biomembr 49:75–99
Checchetto V, Szabo I (2018) Novel channels of the outer membrane of mitochondria: recent discoveries change our view. BioEssays 40:1700232
Colombini M (2012) VDAC structure, selectivity, and dynamics. Biochim Biophys Acta 1818:1457–1465
Dekker PJ, Ryan MT, Brix J, Müller H, Hönlinger A, Pfanner N (1998) Preprotein translocase of the outer mitochondrial membrane: molecular dissection and assembly of the general import pore complex. Mol Cell Biol 18:6515–6524
Elbaz-Alon Y, Eisenberg-Bord M, Shinder V, Stiller SB, Shimoni E, Wiedemann N, Geiger T, Schuldiner M (2015) Lam6 regulates the extent of contacts between organelles. Cell Rep 12:7–17
Ellenrieder L, Opaliński Ł, Becker L, Krüger V, Mirus O, Straub SP, Ebell K, Flinner N, Stiller SB, Guiard B, Meisinger C, Wiedemann N, Schleiff E, Wagner R, Pfanner N, Becker T (2016) Separating mitochondrial protein assembly and endoplasmic reticulum tethering by selective coupling of Mdm10. Nat Commun 7:13021
Ellenrieder L, Dieterle MP, Doan KN, Mårtensson CU, Floerchinger A, Campo ML, Pfanner N, Becker T (2019) Dual role of mitochondrial porin in metabolite transport across the outer membrane and protein transfer to the inner membrane. Mol Cell 73:1056–1065
Endo T, Yamano K (2009) Multiple pathways for mitochondrial protein traffic. Biol Chem 390:723–730
Gebert N, Gebert M, Oeljeklaus S, von der Malsburg K, Stroud DA, Kulawiak B, Wirth C, Zahedi RP, Dolezal P, Wiese S, Simon O, Schulze-Specking A, Truscott KN, Sickmann A, Rehling P, Guiard B, Hunte C, Warscheid B, van der Laan M, Pfanner N, Wiedemann N (2011) Dual function of Sdh3 in the respiratory chain and TIM22 protein translocase of the mitochondrial inner membrane. Mol Cell 44:811–818
Gerbeth C, Schmidt O, Rao S, Harbauer AB, Mikropoulou D, Opalińska M, Guiard B, Pfanner N, Meisinger C (2013) Glucose-induced regulation of protein import receptor Tom22 by cytosolic and mitochondria-bound kinases. Cell Metab 18:578–587
González-Montoro A, Auffarth K, Hönscher C, Bohnert M, Becker T, Warscheid B, Regiorri F, van der Laan M, Fröhlich F, Ungermann C (2018) Vps39 interacts with Tom40 to establish one of two functionally distinct vacuole-mitochondria contact sites. Dev Cell 45:621–636
Harbauer AB, Opalińska M, Gerbeth C, Herman JS, Rao S, Schönfisch B, Guiard B, Schmidt O, Pfanner N, Meisinger C (2014) Cell cycle-dependent regulation of mitochondrial preprotein translocase. Science 346:1109–1113
Kang Y, Stroud DA, Baker MJ, De Souza DP, Frazier AE, Liem M, Tull D, Mathivanan S, McConville MJ, Thorburn DR, Ryan MT, Stojanovski D (2017) Sengers syndrome-associated mitochondrial acylglycerol kinase is a subunit of the human TIM22 protein import complex. Mol Cell 67:457–475
Kornmann B, Currie E, Collins CR, Schuldiner M, Nunnari J, Weissman JS, Walter P (2009) An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science 325:477–481
Krüger V, Becker T, Becker L, Montilla-Martinez M, Ellenrieder L, Vögtle FN, Meyer H, Ryan M, Wiedemann N, Warscheid B, Pfanner N, Wagner R, Meisinger C (2017) Identification of new channels by systematic analysis of the mitochondrial outer membrane. J Cell Biol 216:3485–3495
Künkele KP, Heins S, Dembowski M, Nargang FE, Benz R, Thieffry M, Walz J, Lill R, Nussberger S, Neupert W (1998) The preprotein translocation channel of the outer membrane of mitochondria. Cell 93:1009–1019
Lahiri S, Chao JT, Tavassoli S, Wong AK, Choudhary V, Young BP, Loewen CJ, Prinz WA (2014) A conserved endoplasmic reticulum membrane protein complex (EMC) facilitates phospholipid transfer from the ER to mitochondria. PLoS Biol 12:e1001969
Lazarou M, Jin SM, Kane LA, Youle RJ (2012) Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev Cell 22:320–333
Makki A, Rada P, Žárský V, Kereïche S, Kováčik L, Novotný M, Jores T, Rapaport D, Tachezy J (2019) Triplet-pore structure of a highly divergent TOM complex of hydrogenosomes in Trichomonas vaginalis. PLoS Biol 17:e3000098
Mehnert CS, Rampelt H, Gebert M, Oeljeklaus S, Schrempp SG, Kochbeck L, Guiard B, Warscheid B, van der Laan M (2014) The mitochondrial ADP/ATP carrier associates with the inner membrane presequence translocase in a stoichiometric manner. J Biol Chem 289:27352–27362
Model K, Prinz T, Ruiz T, Rademacher M, Krimmer T, Kühlbrandt W, Pfanner N, Meisinger C (2002) Protein translocase of the outer mitochondrial membrane: role of import receptors in the structural organization of the TOM complex. J Mol Biol 316:657–666
Morgenstern M, Stiller SB, Lübbert P, Peikert CD, Dannenmaier S, Drepper F, Weill U, Höß P, Feuerstein R, Gebert M, Bohnert M, van der Laan M, Schuldiner M, Schütze C, Oeljeklaus S, Pfanner N, Wiedemann N, Warscheid B (2017) Definition of a high-confidence mitochondrial proteome at quantitative scale. Cell Rep 19:2836–2852
Müller CS, Bildl W, Haupt A, Ellenrieder L, Becker T, Hunte C, Fakler B, Schulte U (2016) Cryo-slicing blue native-mass spectrometry (csBN-MS): a novel technology for high-resolution complexome profiling. Mol Cell Proteomics 15:669–681
Murley A, Sarsa RD, Toulmay A, Yamada J, Prinz WA, Nunnari J (2015) Ltc1 is an ER-localized sterol transporter and a component of ER-mitochondria and ER-vacuole contacts. J Cell Biol 209:539–548
Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM (2012) Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science 337:587–590
Neupert W, Herrmann JM (2007) Translocation of proteins into mitochondria. Annu Rev Biochem 76:723–749
Palmieri F, Pierri CL (2010) Mitochondrial metabolite transport. Essays Biochem 47:37–52
Pascual-Ahuir A, Manzanares-Estreder Timón-Gómez A, Proft M (2018) Ask yeast how to burn your fats: lessons learned from the metabolic adaption to salt stress. Curr Genet 64:63–69
Priesnitz C, Becker T (2018) Pathways to balance mitochondrial translation and protein import. Genes Dev 32:1285–1296
Ryan MT, Müller H, Pfanner N (1999) Functional staging of ADP/ATP carrier translocation across the outer mitochondrial membrane. J Biol Chem 274:20619–20627
Sakaue H, Shiota T, Ishizaka N, Kawano S, Tamura Y, Tan KS, Imai K, Motono C, Hirokawa T, Taki K, Miyata N, Kuge O, Lithgow T, Endo T (2019) Porin associates with Tom22 to regulate the mitochondrial protein gate assembly. Mol Cell 73:1044–1055
Schmidt O, Harbauer AB, Rao S, Eyrich B, Zahedi RP, Stojanovski D, Schönfisch B, Guiard B, Sickmann A, Pfanner N, Meisinger C (2011) Regulation of mitochondrial protein import by cytosolic kinases. Cell 144:227–239
Sherman EL, Go NE, Nargang FE (2005) Functions of the small proteins in the TOM complex of Neurospora crassa. Mol Biol Cell 16:4172–4182
Shiota T, Imai K, Qiu J, Hewitt VL, Tan K, Shen HH, Sakiyama N, Fukasawa Y, Hayat S, Kamiya M, Elofsson A, Tomii K, Horton P, Wiedemann N, Pfanner N, Lithgow T, Endo T (2015) Molecular architecture of the active mitochondrial protein gate. Science 349:1544–1548
van der Laan M, Wiedemann N, Mick DU, Guiard B, Rehling P, Pfanner N (2006) A role for Tim21 in membrane potential-dependent preprotein sorting in mitochondria. Curr Biol 16:2271–2276
Vukotic M, Nolte H, König T, Saita S, Ananjew M, Krüger M, Tatsuta T, Langer T (2017) Acylglycerol kinase mutated in Sengers syndrome is a subunit of the TIM22 protein translocase in mitochondria. Mol Cell 67:471–490
Wiedemann N, Pfanner N (2017) Mitochondrial machineries for protein import and assembly. Annu Rev Biochem 86:685–714
Zhang N, Cao L (2017) Starvation signals in yeast are integrated to coordinate metabolic reprogramming and stress responses to ensure longevity. Curr Genet 63:839–843
Acknowledgements
The work was supported by grants of the Deutsche Forschungsgemeinschaft (BE 4679/2–2), Research Training Group 278002225/RTG 2202 and Germany’s Excellence Strategy (CIBSS-EXC-2189—Project ID 390939984). Work included in this study has also been performed in partial fulfillment of the requirements for the doctoral thesis of K.N.D. at the University of Freiburg.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Doan, K.N., Ellenrieder, L. & Becker, T. Mitochondrial porin links protein biogenesis to metabolism. Curr Genet 65, 899–903 (2019). https://doi.org/10.1007/s00294-019-00965-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-019-00965-z