Abstract
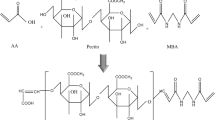
The present research work is focused on development and characterization of copolymerized pectin sulfonic acid hydrogels and to evaluate controlled delivery of captopril. Series of pectin–sulfonic acid-based hydrogels were synthesized by free radical copolymerization technique. Pectin has been chemically cross-linked with 2-acrylamido-2-methylpropane sulfonic acid in the presence of ammonium persulfate and sodium metabisulfite as redox initiator. Methylene bisacrylamide (MBA) was used as cross-linking agent in varying amount to investigate the degree of cross-linking as a function of increased concentration of polymer (pectin) and monomer (AMPS). Captopril was incorporated as model drug in formulated hydrogels. Fourier transform infrared spectroscopy (FTIR) was performed for structural analysis. In vitro swelling and release studies of captopril were carried out at both pH 1.2 and 7.4. Sol–gel fraction was also calculated to determine the amount of uncross-linked polymer fraction in prepared hydrogels. FTIR analysis confirmed the formation of cross-linked network between polymer (pectin) and monomer (AMPS). All formulations showed pH-dependent swelling behavior because of pectin, and drug release pattern was high at pH 1.2, and prolonged release was observed at pH 7.4 because of pH-independent behavior of sulfonic acid. The results of sol–gel analysis confirmed that gel fraction increases as amount of AMPS and MBA was increased. From current research study, it is concluded that stable formulations of pectin–sulfonic acid were formed by optimized copolymerization reaction. This newly developed polymeric network could serve as a potential system for controlled delivery of captopril for prolonged period.









Similar content being viewed by others
References
Nguyen MK, Lee DS (2010) Injectable biodegradable hydrogels. Macromol Biosci 10(6):563–579
Asghar S et al (2018) Hydrophobic–hydrophilic cross-linked matrices for controlled release formulation of highly water-soluble drug venlafaxine: synthesis and evaluation studies. Adv Polym Technol 37(8):3146–3158
Ferfera-Harrar H, Berdous D, Benhalima T (2018) Hydrogel nanocomposites based on chitosan-g-polyacrylamide and silver nanoparticles synthesized using Curcuma longa for antibacterial applications. Polym Bull 75(7):2819–2846
Peppas N et al (2000) Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm 50(1):27–46
Khalid I et al (2018) Preparation and characterization of alginate-PVA-based semi-IPN: controlled release pH-responsive composites. Polym Bull 75(3):1075–1099
Gulrez SK, Phillips GO, Al-Assaf S (2011) Hydrogels: methods of preparation, characterisation and applications. INTECH Open Access Publisher, Climber
Laftah WA, Hashim S, Ibrahim AN (2011) Polymer Hydrogels: a Review. Polym Plast Technol Eng 50(14):1475–1486
Calvo P et al (1997) Novel hydrophilic chitosan polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci 63(1):125–132
Qiu Y, Park K (2012) Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev 64:49–60
Sim S, Figueiras A, Veiga F (2012) Modular hydrogels for drug delivery. J Biomater Nanobiotechnol 3(2):15
Hamidi M, Azadi A, Rafiei P (2008) Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev 60(15):1638–1649
Bhattarai N, Gunn J, Zhang M (2010) Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev 62(1):83–99
Alakhov VY et al (1996) Hypersensitization of multidrug resistant human ovarian carcinoma cells by pluronic P85 block copolymer. Bioconjugate Chem 7(2):209–216
Jeong B, Kim SW, Bae YH (2002) Thermosensitive sol gel reversible hydrogels. Adv Drug Deliv Rev 54(1):37–51
Reimer K et al (1999) An innovative topical drug formulation for wound healing and infection treatment: in vitro and in vivo investigations of a povidone-iodine liposome hydrogel. Dermatology 201(3):235–241
Sri B, Ashok V, Arkendu C (2012) As a review on hydrogels as drug delivery in the pharmaceutical field. Int J Pharm Chem Sci 1(2):642–661
Hickey AS, Peppas NA (1995) Mesh size and diffusive characteristics of semicrystalline poly(vinyl alcohol) membranes prepared by freezing/thawing techniques. J Membr Sci 107(3):229–237
Yoshii E (1997) Cytotoxic effects of acrylates and methacrylates: relationships of monomer structures and cytotoxicity. J Biomed Mater Res 37(4):517–524
Rehmani S et al (2017) Development of natural and synthetic polymer-based semi-interpenetrating polymer network for controlled drug delivery: optimization and in vitro evaluation studies. Polym Bull 74(3):737–761
Sriamornsak P et al (2007) Swelling and erosion of pectin matrix tablets and their impact on drug release behavior. Eur J Pharm Biopharm 67(1):211–219
Thakur BR et al (1997) Chemistry and uses of pectin a review. Crit Rev Food Sci Nutr 37(1):47–73
Sundar Raj A et al (2012) A review on pectin: chemistry due to general properties of pectin and its pharmaceutical uses. Sci Rep 1:550–551
Howes L (1995) Critical assessment of ACE inhibitors. Part 2. Aust Fam Physician 24(4):639–641
Craig CR, Stitzel RE (2004) Modern pharmacology with clinical applications. Lippincott Williams and Wilkins, Philadelphia
Saeb-Parsy K (1999) Instant pharmacology. Wiley, Hoboken
Gad Y (2008) Preparation and characterization of poly (2-acrylamido-2-methylpropane-sulfonic acid)/Chitosan hydrogel using gamma irradiation and its application in wastewater treatment. Radiat Phys Chem 77(9):1101–1107
Raju KN et al (2012) Formulation and in vitro evaluation of buccal tablets of Captopril. Int Res J Pharm Appl Sci 2(2):21–43
Mishra RK, Datt M, Banthia AK (2008) Synthesis and characterization of pectin/PVP hydrogel membranes for drug delivery system. AAPS PharmSciTech 9(2):395–403
Zhang C, Easteal AJ (2003) Study of poly (acrylamide co 2 acrylamido 2 methylpropane sulfonic acid) hydrogels made using gamma radiation initiation. J Appl Polym Sci 89(5):1322–1330
Ranjha NM et al (2010) Preparation and characterization of hybrid pH-sensitive hydrogels of chitosan-co-acrylic acid for controlled release of verapamil. J Mater Sci Mater Med 21(10):2805–2816
Ali L et al (2014) Controlled release of highly water-soluble antidepressant from hybrid copolymer poly vinyl alcohol hydrogels. Polym Bull 71(1):31–46
Durmaz S, Okay O (2000) Acrylamide/2-acrylamido-2-methylpropane sulfonic acid sodium salt-based hydrogels: synthesis and characterization. Polymer 41(10):3693–3704
Kazemzadeh B, Hosseinzadeh H, Babazadeh M (2013) Synthesis of a novel pectin-based superabsorbent hydrogel with salt and pH-responsiveness Properties. Biomed Pharmacol J 6(1):41–48
Dev RK, Bali V, Pathak K (2011) Novel microbially triggered colon specific delivery system of 5-fluorouracil: statistical optimization, in vitro, in vivo, cytotoxic and stability assessment. Int J Pharm 411(1):142–151
Agnihotri SA, Mallikarjuna NN, Aminabhavi TM (2004) Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J Control Release 100(1):5–28
Lee KY, Yuk SH (2007) Polymeric protein delivery systems. Prog Polym Sci 32(7):669–697
Ashford M et al (1993) An evaluation of pectin as a carrier for drug targeting to the colon. J Control Release 26(3):213–220
Hussain T, Ranjha NM, Shahzad Y (2011) Swelling and controlled release of tramadol hydrochloride from a pH-sensitive hydrogel. Des Monomers Polym 14(3):233–249
Acknowledgements
The authors would like to acknowledge financial assistance from the Islamia University of Bahawalpur, Punjab, Pakistan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
khan, Z., Minhas, M.U., Ahmad, M. et al. Functionalized pectin hydrogels by cross-linking with monomer: synthesis, characterization, drug release and pectinase degradation studies. Polym. Bull. 77, 339–356 (2020). https://doi.org/10.1007/s00289-019-02745-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-02745-8