Abstract
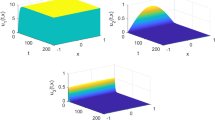
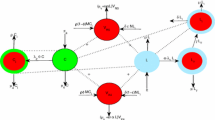
Multiscale models possess the potential to uncover new insights into infectious diseases. Here, a rigorous stability analysis of a multiscale model within-host and between-host is presented. The within-host model describes viral replication and the respective immune response while disease transmission is represented by a susceptible-infected model. The bridging of scales from within- to between-host considered transmission as a function of the viral load. Consequently, stability and bifurcation analyses were developed coupling the two basic reproduction numbers \(R_0^{W}\) and \(R_0^{B}\) for the within- and the between-host subsystems, respectively. Local stability results for each subsystem, including a unique stable equilibrium point, recapitulate classical approaches to infection and epidemic control. Using a Lyapunov function, global stability of the between-host system was obtained. Our main result was the derivation of the \(R_0^{B}\) as an increasing function of \(R_0^{W}\). Numerical analyses reveal that a Michaelis–Menten form based on the virus is more likely to recapitulate the behavior between the scales than a form directly proportional to the virus. Our work contributes basic understandings of the two models and casts light on the potential effects of the coupling function on linking the two scales.








Similar content being viewed by others
References
Alizon S, Luciani F, Regoes RR (2011) Epidemiological and clinical consequences of within-host evolution. Trends Microbiol 19(1):24–32. https://doi.org/10.1016/j.tim.2010.09.005
Anderson RM, May RM (1992) Infectious diseases of humans: dynamics and control. Dynamics and control. Oxford University Press, Oxford
Blaser N, Wettstein C, Estill J, Vizcaya LS, Wandeler G, Egger M, Keiser O (2014) Impact of viral load and the duration of primary infection on HIV transmission: systematic review and meta-analysis. AIDS 28(7):1021–1029. https://doi.org/10.1097/QAD.0000000000000135
Boianelli A, Nguyen VK, Ebensen T, Schulze K, Wilk E, Sharma N, Stegemann-Koniszewski S, Bruder D, Toapanta FR, Guzmán CA et al (2015) Modeling influenza virus infection: a roadmap for influenza research. Viruses 7(10):5274–5304. https://doi.org/10.3390/v7102875
Bokharaie VS, Mason O, Wirth F (2011) Stability and positivity of equilibria for subhomogeneous cooperative systems. Nonlinear Anal 74(17):6416–6426. https://doi.org/10.1016/j.na.2011.06.023
Brauer F, Castillo-Chavez C (2012) Mathematical models for communicable diseases. Society for Industrial and Applied Mathematics, Philadelphia
Cen X, Feng Z, Zhao Y (2014) Emerging disease dynamics in a model coupling within-host and between-host systems. J Theor Biol 361:141–151. https://doi.org/10.1016/S0022-0396(02)00054-2
Communicable Disease Surveillance Centre (1978) Influenza in a boarding school. Br Med J 1(6112):586–590
De la Sen M, Agarwal RP, Ibeas A, Alonso-Quesada S (2010) On a generalized time-varying SEIR epidemic model with mixed point and distributed time-varying delays and combined regular and impulsive vaccination controls. Adv Differ Equ 201(1):281612. https://doi.org/10.1155/2010/281612
Diekmann O, Heesterbeek JAP, Metz JAJ (1990) On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations. J Math Biol 28(4):365–382. https://doi.org/10.1007/BF00178324
Edenborough KM, Gilbertson BP, Brown LE (2012) A mouse model for the study of contact-dependent transmission of influenza A virus and the factors that govern transmissibility. J Virol 86(23):12544–12551. https://doi.org/10.1128/JVI.00859-12
Feng Z, Velasco-Hernandez J, Tapia-Santos B, Leite MCA (2012) A model for coupling within-host and between-host dynamics in an infectious disease. Nonlinear Dyn 68(3):401–411. https://doi.org/10.1007/s11071-011-0291-0
Ferguson NM, Cummings DAT, Cauchemez S, Fraser C, Riley S, Meeyai A, Iamsirithaworn S, Burke DS (2005) Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature 437(7056):209–214. https://doi.org/10.1038/nature04017
Gandolfi A, Pugliese A, Sinisgalli C (2014) Epidemic dynamics and host immune response: a nested approach. J Math Biol 70(3):399–435. https://doi.org/10.1007/s00285-014-0769-8
Gates B (2015) The next epidemic–lessons from Ebola. N Engl J Med 372(15):1381–1384. https://doi.org/10.1056/NEJMp1502918
Gilchrist MA, Coombs D (2006) Evolution of virulence: interdependence, constraints, and selection using nested models. Theor Popul Biol 69(2):145–153. https://doi.org/10.1016/j.tpb.2005.07.002
Gutierrez JB, Galinski MR, Cantrell S, Voit EO (2015) From within host dynamics to the epidemiology of infectious disease: scientific overview and challenges. Math Biosci 270(Part B):143–155. https://doi.org/10.1016/j.mbs.2015.10.002
Handel A, Rohani P (2015) Crossing the scale from within-host infection dynamics to between-host transmission fitness: a discussion of current assumptions and knowledge. Philos Trans R Soc B Biol Sci. https://doi.org/10.1098/rstb.2014.0302
Hatta Y, Hershberger K, Shinya K, Proll SC, Dubielzig RR, Hatta M, Katze MG, Kawaoka Y, Suresh M (2010) Viral replication rate regulates clinical outcome and CD8 T cell responses during highly pathogenic H5N1 influenza virus infection in mice. PLoS Pathog 6(10):e1001139. https://doi.org/10.1371/Journal.ppat.1001139
Heesterbeek H, Anderson RM, Andreasen V, Bansal S, De Angelis D, Dye C, Eames KTD, Edmunds WJ, Frost SDW, Funk S, Hollingsworth TD, House T, Isham V, Klepac P, Lessler J, Lloyd-Smith JO, Metcalf CJE, Mollison D, Pellis L, Pulliam JRC, Roberts MG, Viboud C (2015) Isaac Newton Institute IDD Collaboration: modeling infectious disease dynamics in the complex landscape of global health. Science 347(6227):aaa4339. https://doi.org/10.1126/science.aaa4339
Heffernan JM, Keeling MJ (2009) Implications of vaccination and waning immunity. Proc R Soc B Biol Sci 276(1664):2071–2080. https://doi.org/10.1098/rspb.2009.0057
Hellriegel B (2001) Immunoepidemiology–bridging the gap between immunology and epidemiology. Trends Parasitol 17(2):102–106. https://doi.org/10.1016/s1471-4922(00)01767-0
Hernandez-Vargas EA, Wilk E, Canini L, Toapanta FR, Binder SC, Uvarovskii A, Ross TM, Guzmán CA, Perelson AS, Meyer-Hermann M (2014) Effects of aging on influenza virus infection dynamics. J Virol 88(8):4123–4131. https://doi.org/10.1128/JVI.03644-13
Holling CS (1959) The components of predation as revealed by a study of small-mammal predation of the european pine sawfly. Can Entomol 91(5):293–320. https://doi.org/10.4039/Ent91293-5
Hsu SB (2005) A survey of constructing Lyapunov functions for mathematical models in population biology. Taiwan J Math 9(2):151–173. https://doi.org/10.11650/twjm/1500407791
Legros M, Bonhoeffer S (2016) A combined within-host and between-hosts modelling framework for the evolution of resistance to antimalarial drugs. J R Soc Interface. https://doi.org/10.1098/rsif.2016.0148
Liu X, Stechlinski P (2012) Infectious disease models with time-varying parameters and general nonlinear incidence rate. Appl Math Model 36(5):1974–1994. https://doi.org/10.1016/j.apm.2011.08.019
Longini IM, Nizam A, Xu S, Ungchusak K, Hanshaoworakul W, Cummings DAT, Halloran ME (2005) Containing pandemic influenza at the source. Science 309(5737):1083–1087. https://doi.org/10.1126/science.1115717
Lukens S, DePasse J, Rosenfeld R, Ghedin E, Mochan E, Brown ST, Grefenstette J, Burke DS, Swigon D, Clermont G (2014) A large-scale immuno-epidemiological simulation of influenza A epidemics. BMC Public Health 14(1):267. https://doi.org/10.1186/1471-2458-14-1019
Ma J, Ma Z (2006) Epidemic threshold conditions for seasonally forced SEIR models. Math Biosci Eng 3(1):161–72. https://doi.org/10.3934/mbe.2006.3.161
Mideo N, Alizon S, Day T (2008) Linking within- and between-host dynamics in the evolutionary epidemiology of infectious diseases. Trends Ecol Evol 23(9):511–517. https://doi.org/10.1016/j.tree.2008.05.009
Murillo LN, Murillo MS, Perelson AS (2013) Towards multiscale modeling of influenza infection. J Theor Biol 332:267–290. https://doi.org/10.1016/j.jtbi.2013.03.024
Nguyen VK, Binder SC, Boianelli A, Meyer-Hermann M, Hernandez-Vargas EA (2015) Ebola virus infection modeling and identifiability problems. Front Microbiol 6:7590. https://doi.org/10.3389/fmicb.2015.00257
Nguyen VK, Hernandez-Vargas EA (2017) Windows of opportunity for Ebola virus infection treatment and vaccination. Sci Rep 7(1):8975. https://doi.org/10.1038/s41598-017-08884-0
Saker L, Lee K, Cannito B, Gilmore A, Campbell-Lendrum DH (2004) UNDP/World Bank/WHO special programme for research and training in tropical diseases: globalization and infectious diseases: a review of the linkages. World Health Organization, Geneva
Toapanta FR, Ross TM (2009) Impaired immune responses in the lungs of aged mice following influenza infection. Respir Res 10(1):112. https://doi.org/10.1186/1465-9921-10-112
Wolkowicz GSK, Lu Z (1992) Global dynamics of a mathematical model of competition in the chemostat: general response functions and differential death rates. SIAM J Appl Math 52(1):222–233. https://doi.org/10.1137/0152012
Zhao XQ (2003) Global attractivity in monotone and subhomogeneous almost periodic systems. J Differ Equ 187(2):494–509. https://doi.org/10.1016/S0022-0396(02)00054-2
Zhao XQ, Borwein J, Borwein P (2017) Dynamical systems in population biology. Springer, Berlin
Acknowledgements
We thank the Alfons und Gertrud Kassel-Stiftung for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Almocera, A.E.S., Nguyen, V.K. & Hernandez-Vargas, E.A. Multiscale model within-host and between-host for viral infectious diseases. J. Math. Biol. 77, 1035–1057 (2018). https://doi.org/10.1007/s00285-018-1241-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-018-1241-y