Abstract
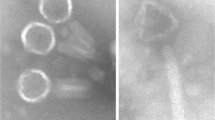
The potential of bacteriophages as alternative treatment for multidrug-resistant (MDR) Klebsiella pneumoniae-related infections has recently gained much interest. The purpose of this research was to isolate and characterize a K. pneumoniae-specific lytic phage with the potential to treat experimental lobar pneumonia induced by K. pneumoniae in mice. A lytic phage was isolated from an urban wastewater sample in Tehran and characterized by transmission electron microscopy (TEM), thermal, pH, and chloroform stability before being employed for treatment of mice infected with K. pneumoniae in an experimental model of lobar pneumonia. BALB/C mice were challenged by intranasal inoculation with 108 colony-forming units (CFU/ml) of K. pneumoniae ATCC 10031 followed by an intraperitoneal injection of the isolated phage using 1010 and 109 plaque-forming units (PFU/ml) simultaneously or 24 h post infection. Control groups of mice received bacteria or bacteriophage alone. Mice were euthanized daily up to 7 days post infection and examined for abnormality in their lungs and livers followed by determining the number of phages and bacteria in plasma and lung homogenates. The isolated phage (vB_KpnM-Teh.1) belonged to the Myoviridae family, was stable at 37 °C, pH 7, and was resistant to chloroform. Treatment of mice with a single dose of phage simultaneously at the time of infection, or 24 h post infection, resulted in seven and five logs decrease of CFU/ml in the lung homogenates up to 3 days after phage administration, respectively. The isolated phage may have the potential as a therapeutic agent against K. pneumoniae infections.





Similar content being viewed by others
References
Paczosa MK, Mecsas J (2016) Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev 80(3):629–661. https://doi.org/10.1128/MMBR.00078-15
Kuehn BM (2013) “Nightmare” bacteria on the rise in US hospitals, long-term care facilities. JAMA 309(15):1573–1574. https://doi.org/10.1001/jama.2013.2922
Henry M, Lavigne R, Debarbieux L (2013) Predicting in vivo efficacy of therapeutic bacteriophages used to treat pulmonary infections. Antimicrob Agents Chemother 57(12):5961–5968. https://doi.org/10.1128/AAC.01596-13
Lin TL, Hsieh PF, Huang YT, Lee WC, Tsai YT, Su PA, Pan YJ, Hsu CR, Wu MC, Wang JT (2014) Isolation of a bacteriophage and its depolymerase specific for K1 capsule of Klebsiella pneumoniae: implication in typing and treatment. J Infect Dis 210(11):1734–1744. https://doi.org/10.1093/infdis/jiu332
Chhibber S, Kaur S, Kumari S (2008) Therapeutic potential of bacteriophage in treating Klebsiella pneumoniae B5055-mediated lobar pneumonia in mice. J Med Microbiol 57(Pt 12):1508–1513. https://doi.org/10.1099/jmm.0.2008/002873-0
Kumari S, Harjai K, Chhibber S (2010) Evidence to support the therapeutic potential of bacteriophage Kpn5 in burn wound infection caused by Klebsiella pneumoniae in BALB/c mice. J Microbiol Biotechnol 20(5):935–941. https://doi.org/10.4014/jmb.0909.09010
Bogovazova GG, Voroshilova NN, Bondarenko VM (1991) The efficacy of Klebsiella pneumoniae bacteriophage in the therapy of experimental Klebsiella infection. Zh Mikrobiol Epidemiol Immunobiol 4:5–8
Drulis-Kawa Z, Mackiewicz P, Kesik-Szeloch A, Maciaszczyk-Dziubinska E, Weber-Dabrowska B, Dorotkiewicz-Jach A, Augustyniak D, Majkowska-Skrobek G, Bocer T, Empel J, Kropinski AM (2011) Isolation and characterisation of KP34–a novel phiKMV-like bacteriophage for Klebsiella pneumoniae. Appl Microbiol Biotechnol 90(4):1333–1345. https://doi.org/10.1007/s00253-011-3149-y
Kęsik-Szeloch A, Drulis-Kawa Z, Weber-Dąbrowska B, Kassner J, Majkowska-Skrobek G, Augustyniak D, Łusiak-Szelachowska M, Żaczek M, Górski A, Kropinski AM (2013) Characterising the biology of novel lytic bacteriophages infecting multidrug resistant Klebsiella pneumoniae. Virol J 10(1):1. https://doi.org/10.1186/1743-422X-10-100
Jin J, Li Z-J, Wang S-W, Wang S-M, Huang D-H, Li Y-H, Ma Y-Y, Wang J, Liu F, Chen X-D (2012) Isolation and characterization of ZZ1, a novel lytic phage that infects Acinetobacter baumannii clinical isolates. BMC Microbiol 12(1):156. https://doi.org/10.1186/1471-2180-12-156
Majdani R (2016) Isolation of lytic bacteriophages against pathogenic Escherichia coli strains in poultry in the northwest of Iran. Arch Razi Inst 71(4):235–244. https://doi.org/10.22034/ari.2016.107508
Kusradze I, Karumidze N, Rigvava S, Dvalidze T, Katsitadze M, Amiranashvili I, Goderdzishvili M (2016) Characterization and testing the efficiency of Acinetobacter baumannii phage vB-GEC_Ab-M-G7 as an antibacterial agent. Front Microbiol 7:1590. https://doi.org/10.3389/fmicb.2016.01590
D’Andrea MM, Marmo P, De Angelis LH, Palmieri M, Ciacci N, Di Lallo G, Demattè E, Vannuccini E, Lupetti P, Rossolini GM (2017) φBO1E, a newly discovered lytic bacteriophage targeting carbapenemase-producing Klebsiella pneumoniae of the pandemic Clonal Group 258 clade II lineage. Sci Rep. https://doi.org/10.1038/s41598-017-02788-9
Dallal MMS, Nikkhahi F, Alimohammadi M, Douraghi M, Rajabi Z, Foroushani AR, Azimi A, Fardsanei F (2019) Phage therapy as an approach to control salmonella enterica serotype enteritidis infection in mice. Rev Soc Bras Med Trop 52:e20190290. https://doi.org/10.1590/0037-8682-0290-2019
Kropinski AM (2018) Practical Advice on the One-Step Growth Curve. Bacteriophages, vol 1681. Springer/Humana Press, New York, pp 41–47. https://doi.org/10.1007/978-1-4939-7343-9_3
Soleimani Sasani M, Eftekhar F, Hosseini M (2019) Isolation and characterization of a Klebsiella pneumoniae specific lytic bacteriophage from a hospital waste-water treatment plant. J Med Microbiol 7(1):6–11. https://doi.org/10.29252/JoMMID.7.1.2.6
Fokine A, Rossmann MG (2014) Molecular architecture of tailed double-stranded DNA phages. Bacteriophage 4(2):e28281. https://doi.org/10.4161/bact.28281
Adriaenssens E, Brister JR (2017) How to name and classify your phage: an informal guide. Viruses 9(4):70. https://doi.org/10.3390/v9040070
Komijani M, Bouzari M, Rahimi F (2016) Detection and characterization of a novel lytic bacteriophage (vB-KpneM-Isf48) against Klebsiella pneumoniae isolates from infected wounds carrying antibiotic-resistance genes (TEM, SHV, and CTX-M). Iran Red Crescent Med J. https://doi.org/10.5812/ircmj.34475(In press)
Paterson DL, Bonomo RA (2005) Extended-spectrum β-lactamases: a clinical update. Clin Microbiol Rev 18(4):657–686. https://doi.org/10.1128/CMR.18.4.657-686.2005
Cao F, Wang X, Wang L, Li Z, Che J, Wang L, Li X, Cao Z, Zhang J, Jin L, Xu Y (2015) Evaluation of the efficacy of a bacteriophage in the treatment of pneumonia induced by multidrug resistance Klebsiella pneumoniae in mice. Biomed Res Int 2015:752930. https://doi.org/10.1155/2015/752930
Malik R, Chhibber S (2009) Protection with bacteriophage KO1 against fatal Klebsiella pneumoniae-induced burn wound infection in mice. J Microbiol Immunol Infect 42(2):134–140
Hung C-H, Kuo C-F, Wang C-H, Wu C-M, Tsao N (2011) Experimental phage therapy in treating Klebsiella pneumoniae-mediated liver abscesses and bacteremia in mice. Antimicrob Agents Chemother 55(4):1358–1365. https://doi.org/10.1128/AAC.01123-10
Morello E, Saussereau E, Maura D, Huerre M, Touqui L, Debarbieux L (2011) Pulmonary bacteriophage therapy on Pseudomonas aeruginosa cystic fibrosis strains: first steps towards treatment and prevention. PLoS ONE 6(2):e16963. https://doi.org/10.1371/journal.pone.0016963
Funding
This work was supported by the Shahid Beheshti Reserch Council (Grant No. 600/1724).
Author information
Authors and Affiliations
Contributions
MSS: designed and performed experiments, analyzed data, and co-wrote the paper. FE: supervised the research, corresponding author.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest associated with this manuscript.
Ethical Approval
This study has been approved by the Animals Ethics Committee at Shahid Beheshti University in Tehran under the reference no. IR.SBU.REC.1398.020.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soleimani Sasani, M., Eftekhar, F. Potential of a Bacteriophage Isolated from Wastewater in Treatment of Lobar Pneumonia Infection Induced by Klebsiella pneumoniae in Mice. Curr Microbiol 77, 2650–2655 (2020). https://doi.org/10.1007/s00284-020-02041-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02041-z