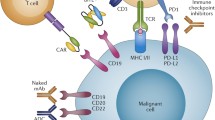
Abstract
Immunotherapy has played an important part in improving the life of patients with lymphoproliferative diseases especially since the addition of rituximab to chemotherapy in the CD20-positive neoplasms in the 1990s. While this field of passive immunotherapy is continuously evolving, several breakthroughs will expand the treatment modalities to include more active immunotherapy. With the approval of immune checkpoint-blocking antibodies for Hodgkin lymphoma and bispecific antibodies for acute lymphoblastic leukemia (ALL), activation of endogenous T cells already plays a role in several lymphoid malignancies. With the approval of cellular therapies with CAR-T cells for ALL and diffuse large B cell lymphoma, the impact of the manipulation of immune responses is taken even further. Vaccines are cellular therapies in the opposite end of the spectrum in terms of side effects, and while the big breakthrough is still to come, the prospect of a very low-toxic immunotherapy which could be applicable also in premalignant states or in frail patients drives a considerable research activity in the area. In this review, we summarize the mechanisms of action and clinical data on trials in the lymphoid neoplasms with chimeric antigen receptor T cells, bispecific antibodies, immune checkpoint-blocking antibodies, and antineoplastic vaccination therapy.

Similar content being viewed by others
References
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, de Moerloose B, Hiramatsu H, Schlis K, Davis KL, Martin PL, Nemecek ER, Yanik GA, Peters C, Baruchel A, Boissel N, Mechinaud F, Balduzzi A, Krueger J, June CH, Levine BL, Wood P, Taran T, Leung M, Mueller KT, Zhang Y, Sen K, Lebwohl D, Pulsipher MA, Grupp SA (2018) Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 378(5):439–448
Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, Roshal M, Maslak P, Davila M, Brentjens RJ, Sadelain M (2018) Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med 378(5):449–459
Fry TJ et al (2018) CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med 24(1):20–28
Porter DL et al (2016) Randomized, phase II dose optimization study of chimeric antigen receptor (CAR) modified T cells directed against CD19 in patients (pts) with relapsed, refractory (R/R) CLL. J Clin Oncol 34:3009–3009
Turtle CJ, Hanafi LA, Berger C, Hudecek M, Pender B, Robinson E, Hawkins R, Chaney C, Cherian S, Chen X, Soma L, Wood B, Li D, Heimfeld S, Riddell SR, Maloney DG (Sep. 2016) Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med 8(355):355ra116
Turtle CJ, Hay KA, Hanafi LA, Li D, Cherian S, Chen X, Wood B, Lozanski A, Byrd JC, Heimfeld S, Riddell SR, Maloney DG (Sep. 2017) Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol 35(26):3010–3020
Gill S et al (2017) CD19 CAR-T cells combined with ibrutinib to induce complete remission in CLL. J Clin Oncol 35:7509–7509
Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera JM, Wei A, Dombret H, Foà R, Bassan R, Arslan Ö, Sanz MA, Bergeron J, Demirkan F, Lech-Maranda E, Rambaldi A, Thomas X, Horst HA, Brüggemann M, Klapper W, Wood BL, Fleishman A, Nagorsen D, Holland C, Zimmerman Z, Topp MS (2017) Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med 376(9):836–847
Ding W, LaPlant BR, Call TG, Parikh SA, Leis JF, He R, Shanafelt TD, Sinha S, le-Rademacher J, Feldman AL, Habermann TM, Witzig TE, Wiseman GA, Lin Y, Asmus E, Nowakowski GS, Conte MJ, Bowen DA, Aitken CN, van Dyke DL, Greipp PT, Liu X, Wu X, Zhang H, Secreto CR, Tian S, Braggio E, Wellik LE, Micallef I, Viswanatha DS, Yan H, Chanan-Khan AA, Kay NE, Dong H, Ansell SM (Apr. 2017) Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood 129(26):3419–3427
Younes A, Brody J, Carpio C, Lopez-Guillermo A, Ben-Yehuda D, Ferhanoglu AB, Nagler A, et al. (2017) Safety and efficacy of the combination of ibrutinib and nivolumab in patients with relapsed non-Hodgkin lymphoma or chronic lymphocytic leukemia. Blood 130(Suppl 1):833
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, Stiff PJ, Friedberg JW, Flinn IW, Goy A, Hill BT, Smith MR, Deol A, Farooq U, McSweeney P, Munoz J, Avivi I, Castro JE, Westin JR, Chavez JC, Ghobadi A, Komanduri KV, Levy R, Jacobsen ED, Witzig TE, Reagan P, Bot A, Rossi J, Navale L, Jiang Y, Aycock J, Elias M, Chang D, Wiezorek J, Go WY (Dec. 2017) Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 377(26):2531–2544
Stephen SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jaeger U, et al. (2017) Primary analysis of Juliet: a global, pivotal, phase 2 trial of CTL019 in adult patients with relapsed or refractory diffuse large B-cell lymphoma. Blood 130(Suppl 1):577
Jeremy AS, Palomba ML, Gordon LI, Lunning MA, Arnason JE, Wang M, Forero A, et al. (2017) High durable CR rates in relapsed/refractory (R/R) aggressive B-NHL treated with the CD19-directed CAR T cell product JCAR017 (TRANSCEND NHL 001): defined composition allows for dose-finding and definition of pivotal cohort. Blood 130(Suppl 1):581
Zhang W et al (2016) Treatment of CD20-directed chimeric antigen receptor-modified T cells in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an early phase IIa trial report. Signal Transduct Target Ther 1(1):16002
Viardot A, Goebeler ME, Hess G, Neumann S, Pfreundschuh M, Adrian N, Zettl F, Libicher M, Sayehli C, Stieglmaier J, Zhang A, Nagorsen D, Bargou RC (2016) Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood 127(11):1410–1416
Goebeler M-E, Knop S, Viardot A, Kufer P, Topp MS, Einsele H, Noppeney R, Hess G, Kallert S, Mackensen A, Rupertus K, Kanz L, Libicher M, Nagorsen D, Zugmaier G, Klinger M, Wolf A, Dorsch B, Quednau BD, Schmidt M, Scheele J, Baeuerle PA, Leo E, Bargou RC (Apr. 2016) Bispecific T-cell engager (BiTE) antibody construct Blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: final results from a phase I study. J Clin Oncol 34(10):1104–1111
Rothe A, Sasse S, Topp MS, Eichenauer DA, Hummel H, Reiners KS, Dietlein M, Kuhnert G, Kessler J, Buerkle C, Ravic M, Knackmuss S, Marschner JP, Pogge von Strandmann E, Borchmann P, Engert A (Jun. 2015) A phase 1 study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory Hodgkin lymphoma. Blood 125(26):4024–4031
Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, Timmerman JM, Collins GP, Ramchandren R, Cohen JB, de Boer JP, Kuruvilla J, Savage KJ, Trneny M, Shipp MA, Kato K, Sumbul A, Farsaci B, Ansell SM (May 2018) Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol 36(14):1428–1439
Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, Radford J, Ribrag V, Molin D, Vassilakopoulos TP, Tomita A, von Tresckow B, Shipp MA, Zhang Y, Ricart AD, Balakumaran A, Moskowitz CH, for the KEYNOTE-087 (Jul. 2017) Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol 35(19):2125–2132
Zinzani PL, Ribrag V, Moskowitz CH, Michot JM, Kuruvilla J, Balakumaran A, Zhang Y, Chlosta S, Shipp MA, Armand P (Jul. 2017) Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood 130(3):267–270
Lesokhin AM, Ansell SM, Armand P, Scott EC, Halwani A, Gutierrez M, Millenson MM, Cohen AD, Schuster SJ, Lebovic D, Dhodapkar M, Avigan D, Chapuy B, Ligon AH, Freeman GJ, Rodig SJ, Cattry D, Zhu L, Grosso JF, Bradley Garelik MB, Shipp MA, Borrello I, Timmerman J (Aug. 2016) Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol 34(23):2698–2704
Kwong Y-L, Chan TSY, Tan D, Kim SJ, Poon LM, Mow B, Khong PL, Loong F, Au-Yeung R, Iqbal J, Phipps C, Tse E (Apr. 2017) PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood 129(17):2437–2442
Freedman A, Neelapu SS, Nichols C, Robertson MJ, Djulbegovic B, Winter JN, Bender JF, Gold DP, Ghalie RG, Stewart ME, Esquibel V, Hamlin P (Jun. 2009) Placebo-controlled phase III trial of patient-specific immunotherapy with mitumprotimut-T and granulocyte-macrophage colony-stimulating factor after rituximab in patients with follicular lymphoma. J Clin Oncol 27(18):3036–3043
Schuster SJ, Neelapu SS, Gause BL, Janik JE, Muggia FM, Gockerman JP, Winter JN, Flowers CR, Nikcevich DA, Sotomayor EM, McGaughey DS, Jaffe ES, Chong EA, Reynolds CW, Berry DA, Santos CF, Popa MA, McCord AM, Kwak LW (Jul. 2011) Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. J Clin Oncol 29(20):2787–2794
Levy R, Ganjoo KN, Leonard JP, Vose JM, Flinn IW, Ambinder RF, Connors JM, Berinstein NL, Belch AR, Bartlett NL, Nichols C, Emmanouilides CE, Timmerman JM, Gregory SA, Link BK, Inwards DJ, Freedman AS, Matous JV, Robertson MJ, Kunkel LA, Ingolia DE, Gentles AJ, Liu CL, Tibshirani R, Alizadeh AA, Denney DW Jr (Jun. 2014) Active idiotypic vaccination versus control immunotherapy for follicular lymphoma. J Clin Oncol 32(17):1797–1803
Garfall AL, Stadtmauer EA, Hwang WT, Lacey SF, Melenhorst JJ, Krevvata M, Carroll MP, et al (2018) Anti-CD19 CAR T cells with high-dose melphalan and autologous stem cell transplantation for refractory multiple myeloma. JCI Insight 3(8):e120505
Ali SA, Shi V, Maric I, Wang M, Stroncek DF, Rose JJ, Brudno JN, Stetler-Stevenson M, Feldman SA, Hansen BG, Fellowes VS, Hakim FT, Gress RE, Kochenderfer JN (2016) T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 128(13):1688–1700
Cohen AD et al (2016) B-cell maturation antigen (BCMA)-specific chimeric antigen receptor T cells (CART-BCMA) for multiple myeloma (MM): initial safety and efficacy from a phase I study. Blood 128(22):1147
Cohen AD et al (2017) Safety and efficacy of B-cell maturation antigen (BCMA)-specific chimeric antigen receptor T cells (CART-BCMA) with cyclophosphamide conditioning for refractory multiple myeloma (MM). Blood 130(Suppl 1):505
Berdeja JG, Lin Y, Raje N, Munshi N, Siegel D, Liedtke M, Jagannath S, Maus MV, Turka A, Ping Lam A, Hege K, Morgan RA, Quigley MT, Kochenderfer JN (2017) Durable clinical responses in heavily pretreated patients with relapsed/ refractory multiple myeloma: updated results from a multicenter study of Bb2121 anti-Bcma CAR T cell therapy. Blood 130(Suppl 1):740
Fan FX et al (2017) Durable remissions with BCMA-specific chimeric antigen receptor (CAR)-modified T cells in patients with refractory/relapsed multiple myeloma. J Clin Oncol 35(18_suppl):LBA3001
Rapoport AP, Stadtmauer EA, Binder-Scholl GK, Goloubeva O, Vogl DT, Lacey SF, Badros AZ et al (2015) NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific nntitumor effects in myeloma. Nat Med 21(8):914–921
Badros A, Hyjek E, Ma N, Lesokhin A, Dogan A, Rapoport AP, Kocoglu M, Lederer E, Philip S, Milliron T, Dell C, Goloubeva O, Singh Z (2017) Pembrolizumab, pomalidomide, and low-dose dexamethasone for relapsed/refractory multiple myeloma. Blood 130(10):1189–1197
Jung S-H, Lee HJ, Lee YK, Yang DH, Kim HJ, Rhee JH, Emmrich F, Lee JJ (2017) A phase I clinical study of autologous dendritic cell therapy in patients with relapsed or refractory multiple myeloma. Oncotarget 8(25):41538–41548
Lacy MQ, Mandrekar S, Dispenzieri A, Hayman S, Kumar S, Buadi F, Dingli D, Litzow M, Wettstein P, Padley D, Kabat B, Gastineau D, Rajkumar SV, Gertz MA (Dec. 2009) Idiotype-pulsed antigen-presenting cells following autologous transplantation for multiple myeloma may be associated with prolonged survival. Am J Hematol 84(12):799–802
Rosenblatt J, Vasir B, Uhl L, Blotta S, Macnamara C, Somaiya P, Wu Z et al (2011) Vaccination with dendritic cell/tumor fusion cells results in cellular and humoral antitumor immune responses in patients with multiple myeloma. Blood 117(2):393–402
Rosenblatt J, Avivi I, Vasir B, Uhl L, Munshi NC, Katz T, Dey BR et al (2013) Vaccination with dendritic cell/tumor fusions following autologous stem cell transplant induces immunologic and clinical responses in multiple myeloma patients. Clinical cancer research: An Official Journal of the American Association for Cancer Research 19(13):3640–3648
June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC (2018) CAR T cell immunotherapy for human cancer. Science 359(6382):1361–1365
Brudno JN, Kochenderfer JN (Aug. 2017) Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol 15(1):31–46
Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, Brogdon JL, Pruteanu-Malinici I, Bhoj V, Landsburg D, Wasik M, Levine BL, Lacey SF, Melenhorst JJ, Porter DL, June CH (Dec. 2017) Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 377(26):2545–2554
Kochenderfer JN, Somerville RPT, Lu T, Shi V, Bot A, Rossi J, Xue A, Goff SL, Yang JC, Sherry RM, Klebanoff CA, Kammula US, Sherman M, Perez A, Yuan CM, Feldman T, Friedberg JW, Roschewski MJ, Feldman SA, McIntyre L, Toomey MA, Rosenberg SA (Jun. 2017) Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol 35(16):1803–1813
Fraietta JA, Beckwith KA, Patel PR, Ruella M, Zheng Z, Barrett DM, Lacey SF, Melenhorst JJ, McGettigan SE, Cook DR, Zhang C, Xu J, Do P, Hulitt J, Kudchodkar SB, Cogdill AP, Gill S, Porter DL, Woyach JA, Long M, Johnson AJ, Maddocks K, Muthusamy N, Levine BL, June CH, Byrd JC, Maus MV (Mar. 2016) Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood 127(9):1117–1127
Cooper ML, Choi J, Staser KW, Ritchey J, Niswonger J, Eckardt K, Rettig MP, et al (2017) An ‘off-the-Shelf’ fratricide-resistant CAR-T for the treatment of T cell hematologic malignancies. Blood 130(Suppl 1):844
Ramos CA, Ballard B, Liu E, Dakhova O, Mei Z, Liu H, Grilley B, et al (2015) Chimeric T cells for therapy of CD30+ Hodgkin and non-Hodgkin lymphomas. Blood 126(23):185
Wang C-M, Wu ZQ, Wang Y, Guo YL, Dai HR, Wang XH, Li X, Zhang YJ, Zhang WY, Chen MX, Zhang Y, Feng KC, Liu Y, Li SX, Yang QM, Han WD (Mar. 2017) Autologous T cells expressing CD30 chimeric antigen receptors for relapsed or refractory Hodgkin lymphoma: an open-label phase I trial. Clin Cancer Res 23(5):1156–1166
Swerdlow SH et al (2017) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, revised 4t, Lyon
Paiva B et al (Feb. 2017) Differentiation stage of myeloma plasma cells: biological and clinical significance. Leukemia 31(2):382–392
Darce JR, Arendt BK, Wu X, Jelinek DF (2007) Regulated expression of BAFF-binding receptors during human B cell differentiation. J Immunol 179(11):7276–7286
Tai YT, Mayes PA, Acharya C, Zhong MY, Cea M, Cagnetta A, Craigen J, Yates J, Gliddon L, Fieles W, Hoang B, Tunstead J, Christie AL, Kung AL, Richardson P, Munshi NC, Anderson KC (2014) Novel anti-B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood 123(20):3128–3138
Seckinger A, Delgado JA, Moser S, Moreno L, Neuber B, Grab A, Lipp S, Merino J, Prosper F, Emde M, Delon C, Latzko M, Gianotti R, Lüoend R, Murr R, Hosse RJ, Harnisch LJ, Bacac M, Fauti T, Klein C, Zabaleta A, Hillengass J, Cavalcanti-Adam EA, Ho AD, Hundemer M, San Miguel JF, Strein K, Umaña P, Hose D, Paiva B, Vu MD (2017) Target expression, generation, preclinical activity, and pharmacokinetics of the BCMA-T cell bispecific antibody EM801 for multiple myeloma treatment. Cancer Cell 31(3):396–410
Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S, Gress RE, Hakim FT, Kochenderfer JN (2013) B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res 19(8):2048–2060
Raje N et al (2018) bb2121 anti-BCMA CAR T-cell therapy in patients with relapsed/refractory multiple myeloma: updated results from a multicenter phase I study. J Clin Oncol 36:abst 8007
Mastaglio S, Genovese P, Magnani Z, Ruggiero E, Landoni E, Camisa B, Schiroli G, Provasi E, Lombardo A, Reik A, Cieri N, Rocchi M, Oliveira G, Escobar G, Casucci M, Gentner B, Spinelli A, Mondino A, Bondanza A, Vago L, Ponzoni M, Ciceri F, Holmes MC, Naldini L, Bonini C (2017) NY-ESO-1 TCR single edited stem and central memory T cells to treat multiple myeloma without graft-versus-host disease. Blood 130(5):606–618
Viardot A, Bargou R (2018) Bispecific antibodies in haematological malignancies. Cancer Treat Rev 65:87–95
Gökbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C, Diedrich H, Topp MS, Brüggemann M, Horst HA, Havelange V, Stieglmaier J, Wessels H, Haddad V, Benjamin JE, Zugmaier G, Nagorsen D, Bargou RC (Apr. 2018) Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood 131(14):1522–1531
von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, Bader P, O’Brien MM, Brethon B, Bhojwani D, Schlegel PG, Borkhardt A, Rheingold SR, Cooper TM, Zwaan CM, Barnette P, Messina C, Michel G, DuBois SG, Hu K, Zhu M, Whitlock JA, Gore L (Dec. 2016) Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol 34(36):4381–4389
Robinson HR, Qi J, Baskar S, Cook E, Ahn IE, Herman SEM, Rader C, Wiestner A (2017) Activity of CD19/CD3 bispecific antibodies in chronic lymphocytic leukemia. Blood 130(Suppl 1):799
Hipp S, Tai YT, Blanset D, Deegen P, Wahl J, Thomas O, Rattel B, Adam PJ, Anderson KC, Friedrich M (Aug. 2017) A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia 31(8):1743–1751
de Zafra C, Balazs M, Fajardo F, Liang L, Zhong W, Henn A, Bernett MJ, et al (2017) Preclinical characterization of AMG 424, a novel humanized T cell-recruiting bispecific anti-CD3/CD38 antibody. Blood 130(Suppl 1):500
Chu SY, Miranda Y, Phung S, Chen H, Rashid R, Endo NA, Chan ET, et al (2014) Immunotherapy with long-lived anti-CD38 × anti-CD3 bispecific antibodies stimulates potent T cell-mediated killing of human myeloma cell lines and CD38+ cells in monkeys: a potential therapy for multiple myeloma. Blood 124(21):4727
Moore GL, Lee SH, Schubbert S, Miranda Y, Rashid R, Pong E, Phung S, et al (2015) Tuning T cell affinity improves efficacy and safety of anti-CD38 × anti-CD3 bispecific antibodies in monkeys - a potential therapy for multiple myeloma. Blood 126(23):1798
Ok CY, Young KH (Dec. 2017) Checkpoint inhibitors in hematological malignancies. J Hematol Oncol 10(1):103
Maude SL, Hucks GE, Seif AE, Talekar MK, Teachey DT, Baniewicz D, Callahan C, et al (2017) The effect of pembrolizumab in combination with CD19-targeted chimeric antigen receptor (CAR) T cells in relapsed acute lymphoblastic leukemia (ALL). J Clin Oncol 35(15):103
Chan TSY, Sim JPY, Kwong Y-L (Sep. 2017) Low-dose nivolumab-induced responses in acute lymphoblastic leukaemia relapse after allogeneic haematopoietic stem cell transplantation. Ann Hematol 96(9):1569–1572
Suen H, Brown R, Yang S, Weatherburn C, Ho PJ, Woodland N, Nassif N, Barbaro P, Bryant C, Hart D, Gibson J, Joshua D (Aug. 2016) Multiple myeloma causes clonal T-cell immunosenescence: identification of potential novel targets for promoting tumour immunity and implications for checkpoint blockade. Leukemia 30(8):1716–1724
Görgün G, SamurMK, Cowens KB, Paula S, Bianchi G, Anderson JE, White RE, et al (2015) Lenalidomide enhances immune checkpoint blockade-induced immune response in multiple myeloma. Clin Cancer Res 21(20):4617–18
Mateos M-V et al (2016) Pembrolizumab in combination with lenalidomide and low-dose dexamethasone for relapsed/refractory multiple myeloma (RRMM): final efficacy and safety analysis. |2016 ASCO Annual Meeting|Abstracts|eeting Library, J Clin Oncol (ASCO Anu. Meet.), vol 34, p. 34: abstr 8010
Pianko MJ, Funt SA, Page DB, Cattry D, Scott EC, Ansell SM, Borrello IM, Gutierrez M, Lendvai N, Hassoun H, Landgren CO, Lesokhin AM (2018) Efficacy and toxicity of therapy immediately after treatment with nivolumab in relapsed multiple myeloma. Leuk Lymphoma 59(1):221–224
Nahas MR, Rosenblatt J, Lazarus HM, Avigan D (2018) Anti-cancer vaccine therapy for hematologic malignancies: an evolving era. Blood Rev 32(4):312–25
Andersen MH (Sep. 2015) Immune regulation by self-recognition: novel possibilities for anticancer immunotherapy. J Natl Cancer Inst 107(9):djv154
Di Nicola M et al (2009) Vaccination with autologous tumor-loaded dendritic cells induces clinical and immunologic responses in indolent B-cell lymphoma patients with relapsed and measurable disease: a pilot study. Blood 113(1):18–27
Maier T, Tun-Kyi A, Tassis A, Jungius KP, Burg G, Dummer R, Nestle FO (Oct. 2003) Vaccination of patients with cutaneous T-cell lymphoma using intranodal injection of autologous tumor-lysate-pulsed dendritic cells. Blood 102(7):2338–2344
Burkhardt UE, Hainz U, Stevenson K, Goldstein NR, Pasek M, Naito M, Wu D, Ho VT, Alonso A, Hammond NN, Wong J, Sievers QL, Brusic A, McDonough SM, Zeng W, Perrin A, Brown JR, Canning CM, Koreth J, Cutler C, Armand P, Neuberg D, Lee JS, Antin JH, Mulligan RC, Sasada T, Ritz J, Soiffer RJ, Dranoff G, Alyea EP, Wu CJ (Sep. 2013) Autologous CLL cell vaccination early after transplant induces leukemia-specific T cells. J Clin Invest 123(9):3756–3765
Yousef S, Marvin J, Steinbach M, Langemo A, Kovacsovics T, Binder M, Kröger N, Luetkens T, Atanackovic D (2015) Immunomodulatory molecule PD-L1 is expressed on malignant plasma cells and myeloma-propagating pre-plasma cells in the bone marrow of multiple myeloma patients. Blood Cancer J 5(3):e285
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a contribution to the special issue on Anti-cancer Immunotherapy: Breakthroughs and Future Strategies -- Guest Editor: Mads Hald Andersen
Electronic supplementary material
ESM 1
(SVG 779 kb)
Rights and permissions
About this article
Cite this article
Klausen, U., Jørgensen, N.G.D., Grauslund, J.H. et al. Cancer immune therapy for lymphoid malignancies: recent advances. Semin Immunopathol 41, 111–124 (2019). https://doi.org/10.1007/s00281-018-0696-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-018-0696-7