Abstract
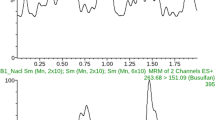
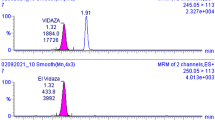
It is desirable to develop a fast method for quantification of melphalan due to its instability. Here we report a method for quantification of melphalan (MPL) in human plasma using a UPLC–PDA system. Briefly, 50 µL plasma sample was mixed with 25 µL internal standard (2500 ng/mL acetylmelphalan in methanol) and 25 µL 20% trichloroacetic acid, and centrifuged at 21,000 g (15,000 rpm) at 4 °C for 3 min. The supernatant (5 µL) was injected onto an Acquity™ BEH C18 LC column (2.1 × 50 mm, 1.7 µm) and eluted with 25 mM NH4AC (pH 4.7)—acetonitrile in a gradient mode at a flow rate of 0.6 mL/min. The column kept at 40 ± 5 °C and the autosampler kept at 4 ± 5 °C. The detector set at 261 nm, and sampling rate was 40points/sec. The retention times were typically 2.11 min for melphalan and 2.38 min for the internal standard. Total run time is 4 min per sample. Calibration range was 100–40,000 ng/mL. The lower limit of quantification was 100 ng/mL. The method was validated based on the FDA guidelines, and applied to a clinical pharmacokinetic study in pediatric patients.


Similar content being viewed by others
References
Krumbhaar EB, Krumbhaar HD (1919) The blood and bone marrow in Yelloe Cross Gas (Mustard Gas) poisoning: changes produced in the bone marrow of fatal cases. J Med Res 40(3):497–508 493
Hall AG, Tilby MJ (1992) Mechanisms of action of, and modes of resistance to, alkylating agents used in the treatment of haematological malignancies. Blood Rev 6(3):163–173
Mizuno K, Dong M, Fukuda T et al (2018) Population pharmacokinetics and optimal sampling strategy for model-based precision dosing of melphalan in patients undergoing hematopoietic stem cell transplantation. Clin Pharmacokinet 57(5):625–636
Bergel F, Stock JA (1954) Cyto-active amino-acid and peptide derivatives. Part I. Substituted phenylalanines. J Chem Soc 0(0):2409–2417
Boschmans J, De Bruijn E, Van Schil P, Lemiere F (2013) Analysis of novel melphalan hydrolysis products formed under isolated lung perfusion conditions using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 27(7):835–841
Ehrsson H, Lonroth U (1982) Degradation of melphalan in aqueous solutions—influence of human albumin binding. J Pharm Sci 71(7):826–827
Reece PA, Hill HS, Green RM et al (1988) Renal clearance and protein binding of melphalan in patients with cancer. Cancer Chemother Pharmacol 22(4):348–352
Nath CE, Shaw PJ, Montgomery K, Earl JW (2007) Population pharmacokinetics of melphalan in paediatric blood or marrow transplant recipients. Br J Clin Pharmacol 64(2):151–164
Gouyette A, Hartmann O, Pico JL (1986) Pharmacokinetics of high-dose melphalan in children and adults. Cancer Chemother Pharmacol 16(2):184–189
Nath CE, Trotman J, Tiley C et al (2016) High melphalan exposure is associated with improved overall survival in myeloma patients receiving high dose melphalan and autologous transplantation. Br J Clin Pharmacol 82(1):149–159
Nath CE, Shaw PJ, Trotman J et al (2010) Population pharmacokinetics of melphalan in patients with multiple myeloma undergoing high dose therapy. Br J Clin Pharmacol 69(5):484–497
Nath CE, Shaw PJ, Montgomery K, Earl JW (2005) Melphalan pharmacokinetics in children with malignant disease: influence of body weight, renal function, carboplatin therapy and total body irradiation. Br J Clin Pharmacol 59(3):314–324
Pinguet F, Joulia JM, Martel P, Grosse PY, Astre C, Bressolle F (1996) High-performance liquid chromatographic assay for melphalan in human plasma. Application to pharmacokinetic studies. J Chromatogr B Biomed Appl 686(1):43–49
Poujol S, Pinguet F, Mougenot P et al (2004) Quantitation of melphalan in human plasma by liquid chromatography and solid phase extraction: Application to pharmacokinetic study. J Liq Chromatogr R T 27(2):301–313
Springolo V, Borella F, Finardi GP, Gatti MT, Coppi G (1989) High-performance liquid chromatographic determination of m-bis(chloroethyl)aminophenyl-l-alanine in plasma. J Chromatogr 490(1):224–229
Chang SY, Alberts DS, Melnick LR, Walson PD, Salmon SE (1978) High-pressure liquid chromatographic analysis of melphalan in plasma. J Pharm Sci 67(5):679–682
Huang L, Lizak P, Dvorak CC, Aweeka F, Long-Boyle J (2014) Simultaneous determination of fludarabine and clofarabine in human plasma by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 960:194–199
Clinical Pharmacology Quanlity Assurance (Cpqa) Program. CPQA Guidelines for chromatographic method development and validation based on (and including) FDA guidelines dated May 2001, Version 4.0 (2012). 1–52 (2012)
Us Department of Health and Human Services, Food and Drug Administration. Guidance for industry: bioanalytical method validation. May 2001
Sparidans RW, Martens I, Valkenburg-Van Iersel LB, Den Hartigh J, Schellens JH, Beijnen JH (2011) Liquid chromatography-tandem mass spectrometric assay for the PARP-1 inhibitor olaparib in combination with the nitrogen mustard melphalan in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 879(21):1851–1856
Huang L, Marzan F, Jayewardene AL, Lizak PS, Li X, Aweeka FT (2009) Development and validation of a hydrophilic interaction liquid chromatography-tandem mass spectrometry method for determination of isoniazid in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 877(3):285–290
Acknowledgements
This work was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131 (a shared instrument award to Dr. Huang) and UCSF-CTSI Grant number KL2 TR000143 (Dr. Long-Boyle). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, L., Cheah, V., Chan, D. et al. Determination of melphalan in human plasma by UPLC–UV method. Cancer Chemother Pharmacol 83, 905–910 (2019). https://doi.org/10.1007/s00280-019-03786-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03786-6