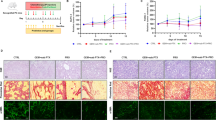
Abstract
Background and aims
Pancreatic ductal adenocarcinoma (PDAC) represents the fourth cause of cancer-related death. We aimed to evaluate whether gemcitabine treatment shapes the gut microbiota in a model of PDAC xenografted mice.
Materials and methods
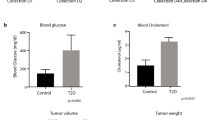
Pancreatic cancer xenograft mice were subjected to gemcitabine injection once per week for 3 weeks to assess the tumor volume as compared to control mice injected with normal saline solution. The composition of fecal microbiota, the activation of NF-kB pathway in cancer tissues and the serum metabolomics were further analyzed.
Results
Gemcitabine considerably decreases the proportion of Gram- positive Firmicutes (from about 39 to 17%) and the Gram- negative Bacteroidetes (from 38 to 17%) which are the two dominant phyla in the gut of tumor-bearing control mice. This downshift was replaced by an increase of Proteobacteria (Escherichia coli and Aeromonas hydrophila) from 15 up to 32% and Verrucomicrobia (Akkermansia muciniphila) from 5 to 33% in the gut of drug-receiving mice. An overall increase in inflammation-associated bacteria was observed upon gemcitabine. Consistently, activation of the NF-kB canonical pathway was found in cancer tissues from gemcitabine-treated mice. Serum metabolomics revealed a significant decrease of the purine compounds inosine and xanthine, and a decreasing trend for their metabolically-related molecule hypoxanthine.
Discussion
Understanding chemotherapy side effects may explain the lack of activity or the chemoresistant processes and it may help to set up strategies to improve the effectiveness of therapy.



Similar content being viewed by others
References
Cid-Arregui A, Juarez V (2015) Perspectives in the treatment of pancreatic adenocarcinoma. World J Gastroenterol 21(31):9297–9316
Conroy T, Bachet JB, Ayav A, Huguet F, Lambert A, Caramella C, Marechal R, Van Laethem JL, Ducreux M (2016) Current standards and new innovative approaches for treatment of pancreatic cancer. Eur J Cancer 57:10–22
Siegel RL, Miller KD, Jemal A, Cancer statistics (2016) CA Cancer. J Clin 66(1):7–30
Cheema AR, O’Reilly EM (2016) Management of Metastatic Pancreatic Adenocarcinoma. Surg Clin North Am 96(6):1391–1414
D’Aronzo M, Vinciguerra M, Mazza T, Panebianco C, Saracino C, Pereira SP, Graziano P, Pazienza V (2015) Fasting cycles potentiate the efficacy of gemcitabine treatment in in vitro and in vivo pancreatic cancer models. Oncotarget 6(21):18545–18557
Gharibi A, Adamian Y, Kelber JA (2016) Cellular and molecular aspects of pancreatic cancer. Acta Histochem 118(3):305–316
Korkeila EA (2015) Advanced pancreatic cancer—how to choose an adequate treatment option. World J Gastroenterol 21(38):10709–10713
Stathis A, Moore MJ (2010) Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol 7(3):163–172
Greer JB, Whitcomb DC (2009) Inflammation and pancreatic cancer: an evidence-based review. Curr Opin Pharmacol 9(4):411–418
Steele CW, Kaur Gill NA, Jamieson NB, Carter CR (2016) Targeting inflammation in pancreatic cancer: clinical translation. World J Gastrointest Oncol 8(4):380–388
Zambirinis CP, Pushalkar S, Saxena D, Miller G (2014) Pancreatic cancer, inflammation, and microbiome. Cancer J 20(3):195–202
Momi N, Kaur S, Krishn SR, Batra SK (2012) Discovering the route from inflammation to pancreatic cancer. Minerva Gastroenterol Dietol 58(4):283–297
Uomo I, Miraglia S, Pastorello M (2010) Inflammation and pancreatic ductal adenocarcinoma: a potential scenario for novel drug targets. JOP 11(3):199–202
Leal-Lopes C, Velloso FJ, Campopiano JC, Sogayar MC, Correa RG (2015) Roles of Commensal Microbiota in Pancreas Homeostasis and Pancreatic Pathologies. J Diabetes Res. https://doi.org/10.1155/2015/284680
Schwabe RF, Jobin C (2013) The microbiome and cancer. Nat Rev Cancer 13(11):800–812
Blaser MJ (2014) The microbiome revolution. J Clin Invest 124(10):4162–4165
Zitvogel L, Galluzzi L, Viaud S, Vetizou M, Daillere R, Merad M, Kroemer G (2015) Cancer and the gut microbiota: an unexpected link. Sci Transl Med 7(271):271ps271
Panebianco C, Adamberg K, Adamberg S, Saracino C, Jaagura M, Kolk K, Di Chio AG, Graziano P, Vilu R, Pazienza V (2017) Engineered resistant-starch (ERS) diet shapes colon microbiota profile in parallel with the retardation of tumor growth in in vitro and in vivo pancreatic cancer models. Nutrients 9(4). https://doi.org/10.3390/nu9040331
Farrell JJ, Zhang L, Zhou H, Chia D, Elashoff D, Akin D, Paster BJ, Joshipura K, Wong DT (2012) Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 61(4):582–588
Michaud DS, Joshipura K, Giovannucci E, Fuchs CS (2007) A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J Natl Cancer Inst 99(2):171–175
Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S, Le Vacon F, de La Cochetiere MF (2014) Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis - current evidence and potential clinical applications. Aliment Pharmacol Ther 40(5):409–421
Paglia G, Williams JP, Menikarachchi L, Thompson JW, Tyldesley-Worster R, Halldorsson S, Rolfsson O, Moseley A, Grant D, Langridge J, Palsson BO, Astarita G (2014) Ion mobility derived collision cross sections to support metabolomics applications. Anal Chem 86(8):3985–3993
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA (2005) Diversity of the human intestinal microbial flora. Science 308(5728):1635–1638
Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI (2005) Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 102(31):11070–11075
Hausmann S, Kong B, Michalski C, Erkan M, Friess H (2014) The role of inflammation in pancreatic cancer. Adv Exp Med Biol 816:129–151
Kawai T, Akira S (2007) Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med 13(11):460–469
Christian F, Smith EL, Carmody RJ (2016) The regulation of NF-kappaB subunits by phosphorylation. Cells 5(1):12
Clemente JC, Ursell LK, Parfrey LW, Knight R (2012) The impact of the gut microbiota on human health: an integrative view. Cell 148(6):1258–1270
O’Hara AM, Shanahan F (2006) The gut flora as a forgotten organ. EMBO Rep 7(7):688–693
Guinane CM, Cotter PD (2013) Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Ther Adv Gastroenterol 6(4):295–308
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444(7122):1027–1031
Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA et al (2012) Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338(6103):120–123
Shin NR, Whon TW, Bae JW (2015) Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 33(9):496–503
Lavelle A, Lennon G, O’Sullivan O, Docherty N, Balfe A, Maguire A, Mulcahy HE, Doherty G, O’Donoghue D, Hyland J, Ross RP, Coffey JC, Sheahan K, Cotter PD, Shanahan F, Winter DC et al (2015) Spatial variation of the colonic microbiota in patients with ulcerative colitis and control volunteers. Gut 64(10):1553–1561
Matsuoka K, Kanai T (2015) The gut microbiota and inflammatory bowel disease. Semin Immunopathol 37(1):47–55
Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, Reyes JA, Shah SA, LeLeiko N, Snapper SB, Bousvaros A, Korzenik J, Sands BE, Xavier RJ, Huttenhower C (2012) Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol 13(9):R79
Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR (2007) Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 104(34):13780–13785
Ganesh BP, Klopfleisch R, Loh G, Blaut M (2013) Commensal Akkermansia muciniphila exacerbates gut inflammation in Salmonella Typhimurium-infected gnotobiotic mice. PLoS One 8(9):e74963
Berry D, Schwab C, Milinovich G, Reichert J, Ben Mahfoudh K, Decker T, Engel M, Hai B, Hainzl E, Heider S, Kenner L, Muller M, Rauch I, Strobl B, Wagner M, Schleper C et al (2012) Phylotype-level 16S rRNA analysis reveals new bacterial indicators of health state in acute murine colitis. ISME J 6(11):2091–2106
Vital M, Howe AC, Tiedje JM (2014) Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. MBio 5(2):e00889
Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ (2008) Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 27(2):104–119
Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A (2011) Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol 17(12):1519–1528
Chopin V, Toillon RA, Jouy N, Le Bourhis X (2002) Sodium butyrate induces P53-independent, Fas-mediated apoptosis in MCF-7 human breast cancer cells. Br J Pharmacol 135(1):79–86
Gaschott T, Maassen CU, Stein J (2001) Tributyrin, a butyrate precursor, impairs growth and induces apoptosis and differentiation in pancreatic cancer cells. Anticancer Res 21(4A):2815–2819
Scheppach W, Weiler F (2004) The butyrate story: old wine in new bottles? Curr Opin Clin Nutr Metab Care 7(5):563–567
Natoni F, Diolordi L, Santoni C, Gilardini Montani MS (2005) Sodium butyrate sensitises human pancreatic cancer cells to both the intrinsic and the extrinsic apoptotic pathways. Biochim Biophys Acta 1745(3):318–329
Blank-Porat D, Gruss-Fischer T, Tarasenko N, Malik Z, Nudelman A, Rephaeli A (2007) The anticancer prodrugs of butyric acid AN-7 and AN-9, possess antiangiogenic properties. Cancer Lett 256(1):39–48
Ogawa H, Rafiee P, Fisher PJ, Johnson NA, Otterson MF, Binion DG (2003) Sodium butyrate inhibits angiogenesis of human intestinal microvascular endothelial cells through COX-2 inhibition. FEBS Lett 554(1–2):88–94
Zgouras D, Wachtershauser A, Frings D, Stein J (2003) Butyrate impairs intestinal tumor cell-induced angiogenesis by inhibiting HIF-1alpha nuclear translocation. Biochem Biophys Res Commun 300(4):832–838
Farrow B, Rychahou P, O’Connor KL, Evers BM (2003) Butyrate inhibits pancreatic cancer invasion. J Gastrointest Surg 7(7):864–870
van Vliet MJ, Harmsen HJ, de Bont ES, Tissing WJ (2010) The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog 6(5):e1000879
Yutin N, Galperin MY (2013) A genomic update on clostridial phylogeny: Gram-negative spore formers and other misplaced clostridia. Environ Microbiol 15(10):2631–2641
Bien J, Palagani V, Bozko P (2013) The intestinal microbiota dysbiosis and Clostridium difficile infection: is there a relationship with inflammatory bowel disease? Therap Adv Gastroenterol 6(1):53–68
Anand A, Glatt AE (1993) Clostridium difficile infection associated with antineoplastic chemotherapy: a review. Clin Infect Dis 17(1):109–113
Masciullo V, Mainenti S, Lorusso D, Margariti PA, Scambia G (2010) Lethal clostridium difficile colitis associated with paclitaxel and carboplatin chemotherapy in ovarian carcinoma: case report and review of the literature. Obstet Gynecol Int 2010:749789
Raza S, Baig MA, Russell H, Gourdet Y, Berger BJ (2010) Clostridium difficile infection following chemotherapy. Recent Pat Antiinfect Drug Discov 5(1):1–9
Lin XB, Dieleman LA, Ketabi A, Bibova I, Sawyer MB, Xue H, Field CJ, Baracos VE, Ganzle MG (2012) Irinotecan (CPT-11) chemotherapy alters intestinal microbiota in tumour bearing rats. PLoS One 7(7):e39764
Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh AS, Hamilton J, Keefe DM (2009) Gastrointestinal microflora and mucins may play a critical role in the development of 5-fluorouracil-induced gastrointestinal mucositis. Exp Biol Med (Maywood) 234(4):430–441
Montrose DC, Zhou XK, McNally EM, Sue E, Yantiss RK, Gross SS, Leve ND, Karoly ED, Suen CS, Ling L, Benezra R, Pamer EG, Dannenberg AJ (2016) Celecoxib alters the intestinal microbiota and metabolome in association with reducing polyp burden. Cancer Prev Res (Phila) 9(9):721–731
Forsgard RA, Marrachelli VG, Korpela K, Frias R, Collado MC, Korpela R, Monleon D, Spillmann T, Osterlund P (2017) Chemotherapy-induced gastrointestinal toxicity is associated with changes in serum and urine metabolome and fecal microbiota in male Sprague–Dawley rats. Cancer Chemother Pharmacol 80(2):317–332
Daliri EB, Wei S, Oh DH, Lee BH (2017) The human microbiome and metabolomics: current concepts and applications. Crit Rev Food Sci Nutr 57(16):3565–3576
He B, Hoang TK, Wang T, Ferris M, Taylor CM, Tian X, Luo M, Tran DQ, Zhou J, Tatevian N, Luo F, Molina JG, Blackburn MR, Gomez TH, Roos S, Rhoads JM et al (2016) Resetting microbiota by Lactobacillus reuteri inhibits T reg deficiency-induced autoimmunity via adenosine A2A receptors. J Exp Med 214(1):107–123
da Rocha Lapa F, da Silva MD, de Almeida Cabrini D, Santos AR (2012) Anti-inflammatory effects of purine nucleosides, adenosine and inosine, in a mouse model of pleurisy: evidence for the role of adenosine A2 receptors. Purinergic Signal 8(4):693–704
Hasko G, Kuhel DG, Nemeth ZH, Mabley JG, Stachlewitz RF, Virag L, Lohinai Z, Southan GJ, Salzman AL, Szabo C (2000) Inosine inhibits inflammatory cytokine production by a posttranscriptional mechanism and protects against endotoxin-induced shock. J Immunol 164(2):1013–1019
Gomez G, Sitkovsky MV (2003) Differential requirement for A2a and A3 adenosine receptors for the protective effect of inosine in vivo. Blood 102(13):4472–4478
Nguyen TL, Vieira-Silva S, Liston A, Raes J (2015) How informative is the mouse for human gut microbiota research? Dis Model Mech 8(1):1–16
Acknowledgements
We thank Morten Danielsen and the MS-Omics for the excellent technical help. No competing interest is declared.
Funding
This research was supported by the ‘‘Ricerca Corrente RC1703GA31’’ funding granted by the Italian Ministry of Health and by the “5 × 1000” voluntary contributions to our Hospital and also by European Regional Development Fund to Competence Center of Food and Fermentation Technologies (EU48667) and Institutional Research Funding to Tallinn University of Technology (IUT 19-27) of the Estonian Ministry of Education and Research.
Author information
Authors and Affiliations
Contributions
Conceived the study: VP. Designed the experiments: CP, KA, RV and VP. Performed the experiments: CP, MJ, SA and KK. Biostatistics Analysis: MC and AF. Analyzed the data: CP, KA and VP. Contributed reagents/materials/analysis tools: KA, RV and VP. Wrote the paper: CP, KA, AA and VP.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
The animal study was performed in an AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care International) accredited experimental facility. Principles of laboratory animal care were followed for the welfare of animals in experimental neoplasia and protocols were approved by the Institutional Animal Care and Use Committee, with the approval number ANM14-002.
Rights and permissions
About this article
Cite this article
Panebianco, C., Adamberg, K., Jaagura, M. et al. Influence of gemcitabine chemotherapy on the microbiota of pancreatic cancer xenografted mice. Cancer Chemother Pharmacol 81, 773–782 (2018). https://doi.org/10.1007/s00280-018-3549-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3549-0