Abstract
The phase 3 COLUMBA study demonstrated noninferiority of subcutaneous daratumumab (DARA SC) to intravenous daratumumab (DARA IV) in relapsed or refractory multiple myeloma. We present a subgroup analysis of Asian patients from COLUMBA. Eligible patients had ≥ 3 prior lines of therapy, including a proteasome inhibitor and an immunomodulatory drug, or were double refractory. Co-primary endpoints were overall response rate (ORR) and maximum trough concentration (Ctrough). Secondary endpoints included rates of infusion-related reactions, progression-free survival, and patient-reported satisfaction with therapy. Sixty-seven Asian patients (DARA SC, n = 30; DARA IV, n = 37) were randomized, including 42 Japanese patients (DARA SC, n = 18; DARA IV, n = 24). Comparable ORRs for DARA SC versus DARA IV were seen in the Asian cohort (66.7% vs 43.2%) and Japanese-only cohort (61.1% vs 54.2%), including patients weighing ≤ 65 kg. Similarity of Ctrough was seen in both Asian and Japanese-only cohorts; the ratio of the geometric mean of the Ctrough concentrations for DARA SC/DARA IV was 143.96% (90% confidence interval (CI), 112.03–185.00%) and 148.02% (90% CI, 113.32–193.34%), respectively. The Asian cohort (both treatment groups) and Japanese-only cohort (DARA SC group) experienced higher rates of grade 3/4 cytopenias compared with the global COLUMBA population, occurring predominantly in patients of low bodyweight; no patients discontinued treatment due to cytopenias. The Cancer Therapy Satisfaction Questionnaire results generally favored DARA SC. In the Asian and Japanese-only cohorts, DARA SC was comparable to DARA IV. The efficacy, pharmacokinetic, safety, and satisfaction results were generally consistent with the global COLUMBA population regardless of patient bodyweight. ClinicalTrials.gov Identifier: NCT03277105
Similar content being viewed by others
Introduction
Daratumumab is a human IgGκ monoclonal antibody targeting CD38 with a direct on-tumor [1,2,3,4] and immunomodulatory [5,6,7] mechanism of action. Based on the positive efficacy and safety results from phase 1/2 (the GEN501 and SIRIUS studies) [8, 9] and phase 3 (the CASTOR, POLLUX, ALCYONE, MAIA, and CASSIOPEIA studies) [10,11,12,13,14] clinical trials, intravenous daratumumab (DARA IV) 16 mg/kg is approved in many countries as monotherapy and in combination with standard-of-care regimens for newly diagnosed multiple myeloma (NDMM) and relapsed or refractory multiple myeloma (RRMM) [15, 16].
DARA IV has median infusion durations during first, second, and subsequent infusions of 7, 4, and 3 hours, respectively [15]. To reduce the duration of administration of daratumumab, a subcutaneous version of daratumumab (DARA SC) co-formulated with recombinant human hyaluronidase PH20 (rHuPH20; ENHANZE® drug delivery technology, Halozyme, Inc., San Diego, CA, USA) was developed that allows for dosing in 3–5 min [17, 18]. Flat dosing of DARA SC was selected based on population pharmacokinetics (PK) analysis and simulations for daratumumab and published literature that support the use of flat dosing for monoclonal antibodies [18,19,20,21,22]. The phase 1b PAVO study demonstrated that DARA SC 1800-mg flat dose had efficacy and PK comparable with DARA IV and lower rates of infusion-related reactions (IRRs) [17].
COLUMBA is a randomized, open-label, noninferiority, phase 3 study that evaluated the efficacy, PK, and safety of DARA SC versus DARA IV monotherapy in patients with heavily pretreated RRMM [23]. In the primary analysis of COLUMBA at a median follow-up of 7.5 months, DARA SC was noninferior to DARA IV in terms of the predefined noninferiority criteria evaluating the co-primary endpoints of overall response rate (ORR) and DARA maximum trough concentration (Ctrough; pre-dose on cycle 3 day 1) [23]. DARA SC also demonstrated a similar safety profile compared to DARA IV while reducing the median time of treatment administration and the rate of IRRs (12.7% vs 34.5%; P < 0.0001) [23]. With a longer median follow-up (13.7 months), DARA SC maintained noninferiority to DARA IV, and DARA SC continued to have a similar safety profile as DARA IV and shorter median administration duration and a reduction in IRR rates [24].
In Asian (Japanese, Korean, and Taiwanese) patients in the phase 3 POLLUX study of daratumumab in combination with lenalidomide and dexamethasone (D-Rd) or lenalidomide and dexamethasone (Rd) alone in patients with RRMM, D-Rd prolonged progression-free survival (PFS) and increased response rates versus Rd alone, consistent with the findings in the global POLLUX study population [25]. Similarly, consistent efficacy with the full study population was also seen in Asian (Japanese and Korean) patients in the phase 3 ALCYONE study of daratumumab in combination with bortezomib, melphalan, and prednisone (D-VMP) or bortezomib, melphalan, and prednisone (VMP) alone in patients with NDMM who were ineligible for autologous stem cell transplant (ASCT) compared to the results of the global ALCYONE study population [26]. In both studies, no new safety concerns were observed for Asian patients [25, 26].
To characterize DARA SC versus DARA IV in Asian patients with heavily pretreated RRMM, we performed a post hoc analysis of Asian (Japanese, Korean, and Taiwanese) patients enrolled in COLUMBA.
Patients and methods
Patients
A total of 67 Asian patients from the phase 3, randomized, open-label, multicenter, noninferiority COLUMBA study (ClinicalTrials.gov Identifier: NCT03277105) were included in this analysis. A separate subanalysis of the 42 Japanese patients alone was also conducted. An independent ethics committee or institutional review board approved the trial, and all patients provided written informed consent. The study protocol was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation guidelines on Good Clinical Practice.
The complete eligibility criteria have been described previously [23]. Briefly, eligible patients were ≥ 18 years of age, had documented RRMM with measurable disease at screening according to International Myeloma Working Group criteria [27, 28], received ≥ 3 prior lines of therapy (including a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD)) or were refractory to both a PI and an IMiD, achieved at least a partial response (PR) to ≥ 1 prior line of therapy, and had an Eastern Cooperative Oncology Group performance status score of ≤ 2.
Dosing
Patients were randomly assigned (1:1) according to planned stratification factors (bodyweight at baseline (≤ 65 kg, 66–85 kg, > 85 kg), number of prior lines of therapy (≤ 4 prior lines, > 4 prior lines), and type of myeloma (IgG, non-IgG)) [23]. Patients received DARA SC (1800-mg flat dose in a 15-mL solution administered by manual push over 3–5 min at left or right abdominal sites, alternating between individual doses) or DARA IV (16 mg/kg) given in 28-day cycles weekly for cycles 1 and 2, every 2 weeks for cycles 3–6, and every 4 weeks thereafter until disease progression.
Evaluation and statistical analyses
The intent-to-treat (ITT) population included all patients randomized into the study. The PK-evaluable population included patients who received all 8 weekly doses of DARA IV or DARA SC in cycles 1 and 2 at the protocol-defined time points and provided a pre-dose PK sample on cycle 3 day 1. The safety population included all randomized patients who received ≥ 1 dose of daratumumab. The immunogenicity-evaluable population included patients who received ≥ 1 dose of daratumumab and had ≥ 1 serum sample after the start of the first dose of daratumumab.
The co-primary endpoints were ORR, defined as the number and proportion of patients achieving PR or better, and daratumumab maximum Ctrough (pre-dose on cycle 3 day 1). Secondary endpoints included PFS, rates of IRRs, rate of very good partial response (VGPR) or better, rate of complete response (CR) or better, duration of and time to response, and patient-reported satisfaction with therapy. Patient-reported satisfaction with therapy was measured using a modified version of the Cancer Therapy Satisfaction Questionnaire (CTSQ), which included 9 items specific to satisfaction with therapy and a mean domain score that was calculated based on responses to 7 of 9 items [29]. Statistical analyses and additional methods used in COLUMBA were described previously [23] and are described briefly in the Supplementary information.
Exploratory subgroup analyses were conducted in the Asian and Japanese-only cohorts to assess the efficacy, safety, and PK of DARA SC versus DARA IV based on baseline bodyweight.
Results
A total of 522 patients were randomized in COLUMBA; 67 (12.8%) patients were Asian (DARA SC, n = 30; DARA IV, n = 37), including 11 Korean (DARA SC, n = 4; DARA IV, n = 7), 14 Taiwanese (DARA SC, n = 8; DARA IV, n = 6), and 42 Japanese (DARA SC, n = 18; DARA IV, n = 24) patients. Baseline patient demographics, treatment history, and clinical characteristics are summarized in Table 1 and Supplementary Table 1 in the Supplementary information. In the Asian cohort, the median age was 70.0 (range, 33–84) years and the median bodyweight was 57.1 (range, 32.8–93.0) kg. In the Japanese-only cohort, the median age was 70.5 (range, 33–84) years and the median bodyweight was 52.9 (range, 32.8–83.2) kg. Only 1 patient in the Asian cohort (see Supplementary information for patient narrative) and no patients in the Japanese-only cohort had a bodyweight of > 85 kg. The Asian cohort received a median of 3.0 (range, 1–15) prior lines of therapy, and the Japanese-only cohort received a median of 4.0 (range, 1–15) prior lines of therapy. In the Asian cohort, 77.6% of patients were refractory to their last line of prior therapy, and 46.3% were refractory to both a PI and an IMiD. In the Japanese-only cohort, 69.0% of patients were refractory to the last line of prior therapy, and 54.8% were refractory to both a PI and an IMiD. A higher proportion of patients in the DARA SC group had high cytogenetic risk at baseline versus the DARA IV group (Asian, 30.8% vs 21.6%; Japanese-only, 37.5% vs 16.7%, respectively). A higher proportion of patients in the DARA SC group also had baseline grade ≥ 2 anemia and neutropenia (anemia: Asian, 46.7% vs 40.5%; Japanese-only, 55.6% vs 33.3%; neutropenia: Asian, 23.3% vs 16.2%; Japanese-only, 22.2% vs 8.3%, respectively).
In the Asian cohort, 14 (46.7%) patients in the DARA SC group and 23 (62.2%) patients in the DARA IV group had discontinued treatment. The most common reason for discontinuation of treatment was progressive disease (DARA SC, 11 (36.7%) patients; DARA IV, 19 (51.4%) patients). Other reasons for discontinuation of treatment in the Asian cohort were adverse event, patient decision, physician decision (each observed in 1 patient in each group), and death (1 patient in the DARA IV group). In the Japanese-only cohort, 9 (50.0%) patients in the DARA SC group and 13 (54.2%) patients in the DARA IV group had discontinued treatment, most frequently due to progressive disease (DARA SC, 7 (38.9%) patients; DARA IV, 12 (50.0%) patients). Other reasons for discontinuation of treatment in the Japanese-only cohort were death (1 patient in the DARA IV group) and patient decision and physician decision (each observed in 1 patient in the DARA SC group).
In the Asian cohort, the median duration of treatment was 6.5 months in the DARA SC group and 5.6 months in the DARA IV group, and the median number of treatment cycles completed was similar (8.0 vs 6.0, respectively). In the Japanese-only cohort, the median duration of treatment was similar between DARA SC and DARA IV (6.5 months vs 6.4 months, respectively), and a median of 8 treatment cycles was completed in both the DARA SC and DARA IV groups.
In the Asian cohort, the median relative dose intensity of daratumumab was 100% for DARA SC and 99.7% for DARA IV for all treatment cycles. In the Japanese-only cohort, the median relative dose intensity of DARA was 100% for DARA SC and 99.9% for DARA IV for all treatment cycles. Consistent with results from the overall study population, in the Asian and Japanese-only cohorts, the median duration of infusion was reduced with DARA SC versus DARA IV during the first (Asian, 4 min vs 418 min; Japanese-only, 4 min vs 421 min), second (Asian, 4 min vs 260 min; Japanese-only, 4 min vs 261 min), and all subsequent infusions (Asian, 4 min vs 208 min; Japanese-only, 3 min vs 210 min), respectively.
Efficacy
In the Asian cohort, the ORR was 66.7% for DARA SC versus 43.2% for DARA IV (Table 2). When assessed based on baseline bodyweight, ORRs for DARA SC versus DARA IV were 50.0% versus 41.2% in the ≤ 55-kg subgroup, 66.7% versus 35.5% in the ≤ 65-kg subgroup, and 66.7% versus 100% in the > 65–85–kg subgroup. Consistent with the global COLUMBA ITT population, comparable ORRs for DARA SC versus DARA IV were seen (relative risk, 1.54; 95% confidence interval (CI), 0.99–2.46), including in the ≤ 65-kg subgroup (relative risk, 1.88; 95% CI, 1.09–3.35). Rates of CR or better and VGPR or better were improved with DARA SC versus DARA IV, including in the ≤ 65-kg subgroup (Table 2). The median duration of response was not reached (NR) for DARA SC versus 10.4 (95% CI, 8.31–not estimable (NE)) months for DARA IV; similar results were seen in the ≤ 55-kg and ≤ 65-kg subgroups (Table 2).
In the Japanese-only cohort, the ORR was 61.1% for DARA SC versus 54.2% for DARA IV (Supplementary Table 2 in the Supplementary information). ORRs were 45.5% versus 50.0% in the ≤ 55-kg subgroup, 58.8% versus 42.1% in the ≤ 65-kg subgroup, and 100% versus 100% in the > 65–85–kg subgroup. As in the Asian cohort, comparable ORRs for DARA SC versus DARA IV were seen (relative risk, 1.13; 95% CI, 0.64–1.93), including in the ≤ 65-kg subgroup (relative risk, 1.40; 95% CI, 0.72–2.80). Rates of VGPR or better were improved with DARA SC versus DARA IV (Supplementary Table 2). The median durations of response were similar to those reported for the Asian cohort (Table 2 and Supplementary Table 2).
After a median follow-up of 13.7 (range, 0.03–19.4) months for the ITT population, the median PFS in the Asian cohort was 11.1 months with DARA SC versus 6.6 months with DARA IV (hazard ratio (HR), 0.62; 95% CI, 0.32–1.22; P = 0.1612; Fig. 1a); 6-month and 12-month PFS rates were 72.4% versus 50.3% and 46.6% versus 28.3%, respectively. In the ≤ 55-kg subgroup, the median PFS was 6.6 months with DARA SC versus 9.3 months with DARA IV (HR, 1.04; 95% CI, 0.37–2.95; P = 0.9438; Fig. 1b); 6-month and 12-month PFS rates were 63.6% versus 57.0% and 33.9% versus 22.8%, respectively. PFS results in the ≤ 65-kg subgroup were similar to those in the overall Asian cohort (Fig. 1c). In the > 65–85–kg subgroup, the median PFS was 11.1 months with DARA SC versus NR with DARA IV (HR, 1.01; 95% CI, 0.14–7.21; P = 0.9919); 6-month and 12-month PFS rates were 66.7% versus 60.0% and 44.4% versus NE, respectively.
In the Japanese-only cohort, the median PFS was 8.3 months with DARA SC versus 9.3 months with DARA IV (HR, 0.89; 95% CI, 0.36–2.16; P = 0.7870; Supplementary Fig. 1a in the Supplementary information); 6-month and 12-month PFS rates were 70.6% versus 54.2% and 34.3% versus 0%, respectively. In the ≤ 55-kg subgroup, the median PFS was 6.5 months with DARA SC versus 10.3 months with DARA IV (HR, 1.91; 95% CI, 0.53–6.86; P = 0.3122; Supplementary Fig. 1b in the Supplementary information). PFS results in the ≤ 65-kg subgroup were comparable to those in the overall Japanese-only cohort (Supplementary Fig. 1c in the Supplementary information). In the > 65–85–kg subgroup, the median PFS was NR with DARA SC or DARA IV (P = 0.5024); 6-month PFS rates were 100.0% versus 60.0%.
Pharmacokinetics
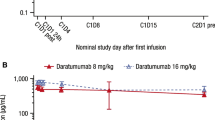
The co-primary endpoint of median maximum Ctrough at cycle 3 day 1 for the DARA SC and DARA IV groups in the Asian cohort was 729 (range, 352–1543) μg/mL and 621 (range, 120–1036) μg/mL, respectively (Fig. 2); the ratio of the geometric mean of the maximum Ctrough concentrations for DARA SC/DARA IV was 143.96% (90% CI, 112.03–185.00%). Consistent with the global COLUMBA PK-evaluable population, a similarity of Ctrough was seen for DARA SC versus DARA IV, with lower bounds of the 90% CI for the ratio of geometric means of Ctrough ≥ 80%. The median maximum Ctrough for the DARA SC and DARA IV groups was 941 (range, 360–1543) μg/mL and 540 (range, 197–883) μg/mL in the ≤ 55-kg subgroup, 803 (range, 352–1543) μg/mL and 540 (range, 120–938) μg/mL in the ≤ 65-kg subgroup, and 526 (range, 378–640) μg/mL and 648 (range, 438–1036) μg/mL in the > 65–85–kg subgroup, respectively (Fig. 2).
Maximum Ctrough on cycle 3 day 1 of Asian patients by bodyweight subgroups. Ctrough, trough concentration; DARA, daratumumab; IV, intravenous; SC, subcutaneous. The boxes represent the 25th, 50th, and 75th percentiles, and the whiskers represent the furthest values from the median that did not exceed 1.5× interquartile range. Data above or below the respective whisker ends displayed as circles are considered outliers. The diamonds inside each box represent the algorithm mean
Similar to the Asian cohort, the median maximum Ctrough at cycle 3 day 1 for the DARA SC and DARA IV groups in the Japanese-only cohort was 814 (range, 360–1543) μg/mL and 592 (range 260–1036) μg/mL, respectively (Supplementary Fig. 2 in the Supplementary information); the ratio of the geometric mean of the maximum Ctrough concentrations for DARA SC/DARA IV was 148.02% (90% CI, 113.32–193.34%), again demonstrating a similarity of Ctrough for DARA SC versus DARA IV. The median maximum Ctrough for the DARA SC and DARA IV groups was 887 (range, 360–1543) μg/mL and 540 (range, 328–883) μg/mL in the ≤ 55-kg subgroup, and 814 (range, 360–1543) μg/mL and 540 (range, 260–883) μg/mL in the ≤ 65-kg subgroup, respectively (Supplementary Fig. 2). The median maximum Ctrough for the 5 PK-evaluable patients in the > 65–85–kg subgroup receiving DARA IV was 648 (range, 438–1036) μg/mL (Supplementary Fig. 2); no patients in this subgroup who received DARA SC were PK evaluable.
Safety
The most common (> 25%) any-grade treatment-emergent adverse events (TEAEs) are summarized in Supplementary Table 3 in the Supplementary information. Although neutropenia was the most common hematologic TEAE in the Asian and Japanese-only cohorts, no treatment discontinuations or deaths occurred due to neutropenia. Febrile neutropenia was only reported with DARA IV in 2 non-Japanese Asian patients in the ≤ 65-kg subgroup.
In the Asian cohort, grade 3/4 TEAEs were reported in 16 (53.3%) patients in the DARA SC group and 21 (56.8%) patients in the DARA IV group (Supplementary Table 4 in the Supplementary information). Patients with baseline bodyweight ≤ 55 kg and ≤ 65 kg had higher rates of grade 3/4 TEAEs compared to those with baseline bodyweight > 65–85 kg (Supplementary Table 4). The most common (> 5%) grade 3/4 TEAEs are summarized in Table 3. The rates of grade 3/4 neutropenia (26.7% for DARA SC and 13.5% for DARA IV, respectively), lymphopenia (13.3% and 8.1%), and leukopenia (6.7% and 2.7%) were higher in the Asian cohort compared to the global COLUMBA safety population, whereas the rates of grade 3/4 anemia were similar (13.3% and 10.8%). Similarly, rates of grade 3/4 neutropenia, lymphopenia, and leukopenia were higher in the ≤ 55-kg and ≤ 65-kg subgroups compared to the global COLUMBA safety population. Rates of grade 3/4 neutropenia were also higher in the > 65–85–kg subgroup compared to the global COLUMBA safety population, whereas no patients in this subgroup experienced grade 3/4 lymphopenia or leukopenia.
In the Japanese-only cohort, grade 3/4 TEAEs were reported in 10 (55.6%) patients in the DARA SC group and 10 (41.7%) patients in the DARA IV group (Supplementary Table 4). As in the Asian cohort, the Japanese-only cohort had high rates of grade 3/4 neutropenia (27.8% for DARA SC and 0% for DARA IV, respectively), lymphopenia (16.7% and 8.3%), and leukopenia (11.1% and 4.2%; Supplementary Table 5 in the Supplementary information). Grade 3/4 anemia was reported at a higher rate with DARA SC (22.2%) compared to the global COLUMBA safety population and occurred in no patients receiving DARA IV. No patients in the > 65–85–kg subgroup experienced a grade 3/4 TEAE.
In the Asian cohort, any-grade infections were reported at similar rates for DARA SC and DARA IV (66.7% vs 67.6%, respectively), and a lower rate of grade 3/4 infections was observed with DARA SC versus DARA IV (3.3% vs 16.2%; Supplementary Table 4). A similar pattern was observed in the ≤ 55-kg and ≤ 65-kg subgroups (Supplementary Table 4). The higher rates of grade 3/4 neutropenia observed with DARA SC versus DARA IV across bodyweight subgroups did not result in higher rates of grade 3/4 infections (Table 3 and Supplementary Table 4). Medication for infection prophylaxis was used in 60% of patients in the DARA SC group and 51.4% of patients in the DARA IV group, with the highest rates of use among patients in the ≤ 55-kg subgroup receiving DARA SC (75.0%; Supplementary Table 4). The most common (> 2 patients in either treatment group) medications for infection prophylaxis were sulfamethoxazole/trimethoprim (33.3% and 21.6% of patients in the DARA SC and DARA IV groups, respectively) and acyclovir (50.0% and 35.1%). No patients receiving DARA SC died as a result of an infection versus 2 (5.4%) patients in the ≤ 65-kg subgroup receiving DARA IV (including 1 patient in the ≤ 55-kg subgroup). Neutropenic sepsis was not reported in any patients.
In the Japanese-only cohort, any-grade and grade 3/4 infections were reported at similar rates for DARA SC versus DARA IV (any grade, 61.1% vs 58.3%; grade 3/4, 5.6% vs 8.3%, respectively; Supplementary Table 4). Rates of any-grade infections were also reported at similar rates for DARA SC versus DARA IV for the ≤ 55-kg and ≤ 65-kg subgroups. Compared with the overall Japanese-only cohort, rates of grade 3/4 infections were higher in the ≤ 55-kg subgroup, while similar rates were observed in the ≤ 65-kg subgroup (Supplementary Table 4). Medication for infection prophylaxis was used in 83.3% of patients in the DARA SC group and 54.2% of patients in the DARA IV group; these medications were used most frequently in the ≤ 55-kg and ≤ 65-kg subgroups (Supplementary Table 4). Consistent with the Asian cohort, the most common (> 2 patients in either treatment group) medications for infection prophylaxis were sulfamethoxazole/trimethoprim (50.0% and 25.0% of patients in the DARA SC and DARA IV groups, respectively) and acyclovir (66.7% and 29.2%). No patients in the DARA SC group died as a result of an infection versus 1 (4.2%) patient in the ≤ 65-kg subgroup receiving DARA IV.
TEAEs leading to treatment discontinuations in the Asian cohort occurred in 1 (3.3%) patient with DARA SC (increased alanine and aspartate aminotransferase) and 1 (2.7%) patient with DARA IV (hepatitis B reactivation); all were in the ≤ 65-kg subgroup. No patients in the Japanese-only cohort discontinued treatment due to TEAEs. TEAEs leading to death in the Asian cohort occurred in 1 (3.3%) patient with DARA SC (general physical health deterioration in a Japanese patient in the ≤ 65-kg subgroup) and 2 (5.4%) patients with DARA IV (hepatitis B reactivation in 1 patient in the ≤ 65-kg subgroup and sepsis in 1 Japanese patient in the ≤ 55-kg subgroup).
In the Asian cohort, serious adverse events (SAEs) occurred in 4 (13.3%) patients in the DARA SC group and 15 (40.5%) patients in the DARA IV group; 1 SAE (sepsis) in the DARA SC group and 3 SAEs (sepsis, hepatitis B reactivation, and pneumonia) in the DARA IV group were considered related to daratumumab (Supplementary Table 4). Lower SAE rates were also seen with DARA SC versus DARA IV in the ≤ 55-kg and ≤ 65-kg subgroups (Supplementary Table 4).
In the Japanese-only cohort, SAEs occurred in 2 (11.1%) patients in the DARA SC group and 7 (29.2%) patients in the DARA IV group; only 1 SAE (sepsis) in each group was considered related to daratumumab (Supplementary Table 4). All patients who reported an SAE in the Japanese-only cohort had a baseline bodyweight of ≤ 65 kg.
In the Asian cohort, the IRR rate was lower with DARA SC versus DARA IV (10.0% vs 18.9%, respectively; odds ratio, 0.48; 95% CI, 0.11–2.03; P = 0.3120; Supplementary Table 4), which was similar to that reported in the global COLUMBA safety population (12.7% vs 34.5%; odds ratio, 0.28; 95% CI, 0.18–0.44; P < 0.0001). The IRR rate was also lower with DARA SC versus DARA IV in the ≤ 55-kg subgroup (16.7% vs 23.5%, respectively) and ≤ 65-kg subgroup (12.5% vs 19.4%). In the > 65–85–kg subgroup, only 1 patient receiving DARA IV experienced an IRR. All IRRs were mild and occurred primarily during the first administration; no grade 3/4 IRRs were reported. Median time to onset of IRRs was 27.5 (range, 3.2–52.0) hours for DARA SC and 1.7 (range, 1.0–24.5) hours for DARA IV (Supplementary Table 4). In the ≤ 55-kg and ≤ 65-kg subgroups, median time to onset of IRRs with DARA SC was longer than and comparable to that in the overall Asian cohort, respectively (Supplementary Table 4).
In the Japanese-only cohort, the IRR rate was the same for patients receiving DARA SC and DARA IV (16.7% vs 16.7%, respectively; Supplementary Table 4). The IRR rate was lower with DARA SC versus DARA IV in the ≤ 55-kg subgroup (18.2% vs 25.0%, respectively) and was similar between DARA SC and DARA IV in the ≤ 65-kg subgroup (17.6% vs 15.8%). Median time to onset of IRRs was 27.5 (range, 3.2–52.0) hours for DARA SC and 1.5 (range, 1.0–24.5) hours for DARA IV (Supplementary Table 4). The 1 Asian patient in the > 65–85–kg subgroup who experienced an IRR with DARA IV was Japanese; the time to IRR onset was 1 hour.
Immunogenicity
No immunogenicity-evaluable patients in the Asian cohort (DARA SC, n = 29; DARA IV, n = 34) were positive for anti-daratumumab antibodies. Two non-Japanese patients in the DARA SC group were positive for anti-rHuPH20 antibodies at baseline, and 1 non-Japanese patient was positive for anti-rHuPH20 antibodies during treatment; all were nonneutralizing.
Patient-reported satisfaction
Overall, in the Asian cohort, patients receiving DARA SC were more satisfied with their cancer therapy than patients receiving DARA IV (Fig. 3). Compliance rates for the modified CTSQ assessments were high in the DARA SC and DARA IV groups (> 90% of patients) of both the Asian and Japanese-only cohorts. In the Asian cohort, the mean scores for the satisfaction with therapy domain were > 70 points (scale: 0–100; higher values indicate greater satisfaction) for DARA SC treatment and > 65 points for DARA IV treatment and were higher for DARA SC compared with DARA IV at all assessed time points. Additionally, more patients reported positive responses to DARA SC versus DARA IV treatment for the following 2 relevant components of the satisfaction with therapy domain that assessed the effect of the route of administration on patient satisfaction with therapy: “satisfied with form of cancer therapy” and “taking cancer therapy as difficult as expected” (Supplementary Figs. 4a and 5a in the Supplementary information). In the Japanese-only cohort, mean scores were similar between the DARA SC and DARA IV groups (Supplementary Figs. 3, 4b, and 5b in the Supplementary information).
Discussion
In both the Asian and Japanese-only cohorts, comparable ORRs and maximum Ctroughs were observed for DARA SC 1800-mg flat dose compared to DARA IV 16 mg/kg. Response rates and PFS were also similar between DARA SC and DARA IV, including in the lower-bodyweight subgroups. Overall, Asian patients receiving DARA SC were generally more satisfied with their cancer therapy compared to Asian patients receiving DARA IV. The results presented here for the Asian and Japanese-only cohorts overall and based on baseline bodyweight are generally consistent with those from the global COLUMBA study population [24]. At a median follow-up of 7.5 months (primary analysis), in the global population of the COLUMBA study, DARA SC was noninferior to DARA IV in terms of ORR (41.1% vs 37.1%, respectively; relative risk, 1.11; 95% CI, 0.89–1.37) and maximum Ctrough (ratio of DARA SC/DARA IV, 107.93%; 90% CI, 95.74–121.67%) [23]. Median PFS was consistent between DARA SC and DARA IV (5.6 months vs 6.1 months, respectively; HR, 0.99; 95% CI, 0.78–1.26; P = 0.93). Additionally, DARA SC demonstrated a similar safety profile compared to DARA IV while reducing the median time of treatment administration and significantly reducing the rate of IRRs [23]. The noninferiority of DARA SC to DARA IV and similar median PFS and safety profile were also seen at a longer median follow-up of 13.7 months, while higher satisfaction with therapy was reported for DARA SC versus DARA IV [24].
Although grade 3/4 cytopenias occurred at higher rates in the Asian cohort for both DARA SC and DARA IV groups compared with the global COLUMBA safety population [24], this did not result in increased rates of severe infections and no patients discontinued treatment or died due to cytopenias, suggesting that they were well managed. Similarly, in the Japanese-only cohort, patients treated with DARA SC experienced higher rates of grade 3/4 cytopenias, but no Japanese patients discontinued treatment due to TEAEs. Grade 3/4 cytopenias in the Asian and Japanese-only cohorts occurred predominantly in patients of lower bodyweight. These results are generally consistent with the Asian and Japanese-only cohort subgroup analyses of ALCYONE, which showed higher rates of grade 3/4 cytopenias in the Asian and Japanese-only cohorts compared with the global ALCYONE safety population in both the D-VMP and VMP treatment groups [26].
The Asian cohort experienced a lower rate of IRRs with DARA SC versus DARA IV, consistent with observations for the global COLUMBA safety population [24]. The rate of IRRs was similar between the DARA SC group and the DARA IV group in the Japanese-only cohort. While the rate of IRRs with DARA SC was similar between the Asian (10.0%) and Japanese-only cohort (16.7%) and the global COLUMBA safety population (12.7%), the IRR rate was lower with DARA IV in the Asian (18.9%) and Japanese-only cohort (16.7%) compared to that of the global COLUMBA safety population (34.5%) and previous DARA IV studies in Asian (40–49.0%) and Japanese-only (35.0–62.5%) patients [25, 26, 30,31,32]. In a phase 1, dose-escalation DARA IV monotherapy study in Japanese patients, IRRs were reported in 4 of 9 (44.4%) patients (1 patient receiving DARA IV 8-mg/kg dose and 3 patients receiving DARA IV 16 mg/kg) [30].
Importantly, although the median baseline bodyweight in the Asian cohort was numerically lower and the median maximum daratumumab Ctrough observed with DARA SC in the Asian cohort was numerically higher than in the global COLUMBA study population, the efficacy and safety of DARA SC among patients in the Asian cohort were generally consistent with those of the global COLUMBA study population [24]. Among non-Asian patients with baseline bodyweight ≤ 55 kg (DARA SC, n = 22; DARA IV, n = 23), the most common (> 15%) grade 3/4 TEAEs with DARA SC and DARA IV were neutropenia (22.7% and 0%, respectively), thrombocytopenia (18.2% and 8.7%), and anemia (9.1% and 26.1%). Among these patients, the rate of IRRs was lower with DARA SC versus DARA IV (18.2% vs 52.2%; odds ratio, 0.20; 95% CI, 0.05–0.79; P = 0.0185). In comparison, higher rates of grade 3/4 neutropenia and lymphopenia were reported with both DARA SC and DARA IV in the ≤ 55-kg Asian cohort compared with the ≤ 55-kg non-Asian cohort, but none led to treatment discontinuation. Although the small patient numbers limit comparisons, these differences in grade 3/4 cytopenia rates may indicate underlying differences between Asian and non-Asian patients of low bodyweight in terms of vulnerability to these events. The rate of IRRs was similarly lower with DARA SC versus DARA IV. These results are aligned with a subgroup analysis of the overall COLUMBA study population by patient bodyweight, which demonstrated that no dose individualization of DARA SC is necessary on the basis of bodyweight based on comparable efficacy and PK results across bodyweights [33].
In conclusion, DARA SC 1800-mg flat dose was comparable to DARA IV 16 mg/kg, with no new safety concerns, in Japanese, Korean, and Taiwanese patients. Although the analyses in this report are limited by small patient numbers, the efficacy and safety of DARA SC in Asian patients overall and of low bodyweight were consistent with those of the global COLUMBA population.
Data availability
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
References
de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, Oomen LA, Peipp M, Valerius T, Slootstra JW, Mutis T, Bleeker WK, Anderson KC, Lokhorst HM, van de Winkel JG, Parren PW (2011) Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 186:1840–1848
Lammerts van Bueren J, Jakobs D, Kaldenhoven N, Roza M, Hiddingh S, Meesters J, Voorhorst M, Gresnigt E, Wiegman L, Buijsse O, Andringa G, Overdijk MB, Doshi P, Sasser K, de Weers M, Parren PWHI (2014) Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood 124:3474
Overdijk MB, Verploegen S, Bogels M, van Egmond M, Lammerts van Bueren JJ, Mutis T, Groen RW, Breij E, Martens AC, Bleeker WK, Parren PW (2015) Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs 7:311–321
Overdijk MB, Jansen JH, Nederend M, Lammerts van Bueren JJ, Groen RW, Parren PW, Leusen JH, Boross P (2016) The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcγ receptor-mediated cross-linking. J Immunol 197:807–813
Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, Syed K, Liu K, van de Donk NWCJ, Weiss BM, Ahmadi T, Lokhorst HM, Mutis T, Sasser AK (2016) Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 128:384–394
Chiu C, Casneuf T, Axel A, Lysaght A, Bald J, Khokhar NZ, Plesner T, Usmani SZ, Goldschmidt H, Ahmadi T, Chan K, Sasser AK (2016) Daratumumab in combination with lenalidomide plus dexamethasone induces clonality increase and T-cell expansion: results from a phase 3 randomized study (POLLUX). Blood 128:4531
Adams HC III, Stevenaert F, Krejcik J, Van der Borght K, Smets T, Bald J, Abraham Y, Ceulemans H, Chiu C, Vanhoof G, Usmani SZ, Plesner T, Lonial S, Nijhof I, Lokhorst HM, Mutis T, van de Donk NWCJ, Sasser AK, Casneuf T (2019) High-parameter mass cytometry evaluation of relapsed/refractory multiple myeloma patients treated with daratumumab demonstrates immune modulation as a novel mechanism of action. Cytometry A 95:279–289
Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, Minnema MC, Lassen U, Krejcik J, Palumbo A, van de Donk NWCJ, Ahmadi T, Khan I, Uhlar CM, Wang J, Sasser AK, Losic N, Lisby S, Basse L, Brun N, Richardson PG (2015) Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med 373:1207–1219
Lonial S, Weiss BM, Usmani S, Singhal S, Chari A, Bahlis N, Belch A, Krishnan A, Vescio R, Mateos MV, Mazumder A, Orlowski RZ, Sutherland H, Blade J, Scott EC, Oriol A, Berdeja JG, Gharibo M, Stevens DA, LeBlanc R, Sebag M, Callander N, Jakubowiak A, White D, De La Rubia J, Richardson PG, Lisby S, Feng H, Uhlar CM, Khan I, Ahmadi T, Voorhees P (2016) Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet 387:1551–1560
Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, Spicka I, Hungria V, Munder M, Mateos MV, Mark TM, Qi M, Schecter J, Amin H, Qin X, Deraedt W, Ahmadi T, Spencer A, Sonneveld P (2016) Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 375:754–766
Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis N, Usmani S, Rabinovic A, Orlowski RZ, Komarnicki M, Suzuki K, Plesner T, Yoo SS, Yehuda DB, Richardson PG, Goldschmidt H, Reece D, Lisby S, Khokhar NZ, O’Rourke DM, Chiu C, Qin X, Guckert M, Ahmadi T, Moreau P (2016) Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 375:1319–1331
Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, Doyen C, Lucio P, Nagy Z, Kaplan P, Pour L, Cook M, Grosicki S, Crepaldi A, Liberati AM, Campbell P, Shelekhova T, Yoon SS, Iosava G, Fujisaki T, Garg M, Chiu C, Wang J, Carson R, Crist W, Deraedt W, Nguyen H, Qi M, San-Miguel J, ALCYONE Trial Investigators (2018) Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med 378:518–528
Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis N, Basu S, Nahi H, Hulin C, Quach H, Goldschmidt H, O’Dwyer M, Perrot A, Venner CP, Weisel K, Mace JR, Raje N, Attal M, Tiab M, Macro M, Frenzel L, Leleu X, Ahmadi T, Chiu C, Wang J, Van Rampelbergh R, Uhlar CM, Kobos R, Qi M, Usmani SZ (2019) Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med 380:2104–2115
Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, Béné MC, Broijl A, Caillon H, Caillot D, Corre J, Delforge M, Dejoie T, Doyen C, Facon T, Sonntag C, Fontan J, Garderet L, Jie KS, Karlin L, Kuhnowski F, Lambert J, Leleu X, Lenain P, Macro M, Mathiot C, Orsini-Piocelle F, Perrot A, Stoppa AM, van de Donk NWCJ, Wuilleme S, Zweegman S, Kolb B, Touzeau C, Roussel M, Tiab M, Marolleau JP, Meuleman N, Vekemans MC, Westerman M, Klein SK, Levin MD, Fermand JP, Escoffre-Barbe M, Eveillard JR, Garidi R, Ahmadi T, Zhuang S, Chiu C, Pei L, de Boer C, Smith E, Deraedt W, Kampfenkel T, Schecter J, Vermeulen J, Avet-Loiseau H, Sonneveld P (2019) Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet 394:29–38
DARZALEX® (daratumumab) injection, for intravenous use [package insert]. Horsham, PA: Janssen Biotech, Inc; 2020
European Medicines Agency. DARZALEX 20 mg/mL concentrate for solution for infusion [summary of product characteristics]. (2016) http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004077/WC500207296.pdf. Accessed 1 June 2020
San-Miguel J, Usmani SZ, Mateos MV, van de Donk N, Kaufman JL, Moreau P, Oriol A, Plesner T, Benboubker L, Liu K, Hellemans P, Masterson T, Clemens PL, Luo M, Farnsworth A, Nahi H, Chari A (2020) Subcutaneous daratumumab in patients with relapsed or refractory multiple myeloma: part 2 of the open-label, multicenter, dose-escalation phase 1b study (PAVO). Haematologica. [Epub ahead of print]
Usmani SZ, Nahi H, Mateos MV, van de Donk N, Chari A, Kaufman JL, Moreau P, Oriol A, Plesner T, Benboubker L, Hellemans P, Masterson T, Clemens PL, Luo M, Liu K, San-Miguel J (2019) Subcutaneous delivery of daratumumab in relapsed or refractory multiple myeloma. Blood 134:668–677
Clemens PL, Xu XS, Luo M, Chari A, Usmani SZ, Mateos MV, van de Donk NWCJ, Kaufman JL, Moreau P, Oriol A, Plesner T, Benboubker L, San-Miguel J, Sun YN, Farnsworth A, Masterson T, Hellemans P, Qi M, Nahi H (2018) Pharmacokinetics (PK) of subcutaneous daratumumab in patients with relapsed or refractory (RR) multiple myeloma (MM): primary clinical pharmacology analysis of the open-label, multicenter, phase 1b study (PAVO). Presented at: the 60th American Society of Hematology (ASH) Annual Meeting & Exposition; December 1–4, 2018; San Diego, California, USA. Abstract 2006
Bai S, Jorga K, Xin Y, Jin D, Zheng Y, Damico-Beyer LA, Gupta M, Tang M, Allison DE, Lu D, Zhang Y, Joshi A, Dresser MJ (2012) A guide to rational dosing of monoclonal antibodies. Clin Pharmacokinet 51:119–135
Wang DD, Zhang S, Zhao H, Men AY, Parivar K (2009) Fixed dosing versus body size–based dosing of monoclonal antibodies in adult clinical trials. J Clin Pharmacol 49:1012–1024
Hendrikx JMA, Haanen JBAG, Voest EE, Schellens JHM, Huitema ADR, Beijnen JH (2017) Fixed dosing of monoclonal antibodies in oncology. Oncologist 22:1212–1221
Mateos MV, Nahi H, Legiec W, Grosicki S, Vorobyev V, Spicka I, Hungria V, Korenkova S, Bahlis N, Flogegard M, Blade J, Moreau P, Kaiser M, Iida S, Laubach J, Magen H, Cavo M, Hulin C, White D, De Stefano V, Clemens PL, Masterson T, Lantz K, O’Rourke L, Heuck C, Qin X, Parasrampuria DA, Yuan Z, Xu S, Qi M, Usmani SZ (2020) Subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma (COLUMBA): a multicentre, open-label, non-inferiority, randomised, phase 3 trial. Lancet Haematol 7:e370–e380
Usmani SZ, Mateos MV, Nahi H, Grosicki S, Vorobyev V, Spicka I, Hungria V, Korenkova S, Flogegard M, Blade J, Moreau P, Kaiser M, Iida S, Laubach J, Masterson T, Lantz K, O’Rourke L, Heuck C, Qin X, Parasrampuria DA, Qi M, Bahlis N (2019) Randomized, open-label, non-inferiority, phase 3 study of subcutaneous (SC) versus intravenous (IV) daratumumab (DARA) administration in patients with relapsed or refractory multiple myeloma: COLUMBA update. Presented at: the 61st American Society of Hematology (ASH) Annual Meeting & Exposition; December 7–10, 2019; Orlando, Florida, USA. Abstract 1865
Suzuki K, Dimopoulos M, Takezako N, Okamoto S, Shinagawa A, Matsumoto M, Kosugi H, Yoon S, Huang S, Qin X, Qi M, Iida S (2018) Daratumumab, lenalidomide, and dexamethasone in East Asian patients with relapsed or refractory multiple myeloma: subgroup analyses of the phase 3 POLLUX study. Blood Cancer J 8:41
Fujisaki T, Ishikawa T, Takamatsu H, Suzuki K, Min CK, Lee JH, Wang J, Carson R, Crist W, Qi M, Nagafuji K (2019) Daratumumab plus bortezomib, melphalan, and prednisone in East Asian patients with non-transplant multiple myeloma: subanalysis of the randomized phase 3 ALCYONE trial. Ann Hematol 98:2805–2814
Durie BGM, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, Ludwig H, Gahrton G, Beksac M, Crowley J, Belch A, Boccadaro M, Cavo M, Turesson I, Joshua D, Vesole D, Kyle R, Alexanian R, Tricot G, Attal M, Merlini G, Powles R, Richardson P, Shimizu K, Tosi P, Morgan G, Rajkumar SV (2006) International uniform response criteria for multiple myeloma. Leukemia 20:1467–1473
Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, Blade J, Richardson P, Orlowski R, Siegel D, Jagannath S, Facon T, Avet-Loiseau H, Lonial S, Palumbo A, Zonder J, Ludwig H, Vesole D, Sezer O, Munshi NC, San Miguel J, International Myeloma Workshop Consensus Panel 1 (2011) Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood 117:4691–4695
Trask PC, Tellefsen C, Espindle D, Getter C, Hsu MA (2008) Psychometric validation of the Cancer Therapy Satisfaction Questionnaire. Value Health 11:669–679
Iida S, Suzuki K, Kusumoto S, Ri M, Tsukada N, Abe Y, Aoki M, Inagaki M (2017) Safety and efficacy of daratumumab in Japanese patients with relapsed or refractory multiple myeloma: a multicenter, phase 1, dose-escalation study. Int J Hematol 106:541–551
Iida S, Ichinohe T, Shinagawa A, Suzuki K, Takezako N, Aoki M (2017) Safety and efficacy of daratumumab in combination with bortezomib and dexamethasone in Japanese patients with relapsed or refractory multiple myeloma. Int J Hematol 107:460–467
Takamatsu H, Iida S, Shibayama H, Shibayama K, Yamazaki H, Suzuki K (2020) Daratumumab, lenalidomide, and dexamethasone in Japanese patients with transplant-ineligible newly diagnosed multiple myeloma: a phase 1b study. Int J Hematol 111:692–701
Mateos MV, Usmani SZ, Grosicki S, Vorobyev V, Spicka I, Hungria V, Korenkova S, Bahlis N, Flogegard M (2019) Randomized, open-label, non-inferiority, phase 3 study of subcutaneous (SC) versus intravenous (IV) daratumumab (DARA) administration in patients (pts) with relapsed or refractory multiple myeloma (RRMM): body weight subgroup analysis of COLUMBA. Presented at: the 61st American Society of Hematology (ASH) Annual Meeting & Exposition; December 7–10, 2019; Orlando, Florida, USA. Abstract 1906
Acknowledgments
The authors thank the patients who participated in this study and their families, as well as the study co-investigators, research nurses, and coordinators at each of the clinical sites. Medical writing and editorial support were provided by Grace Wang, PharmD, of MedErgy.
Funding
This study was sponsored by Janssen Research & Development, LLC. Medical writing and editorial support were funded by Janssen Global Services, LLC.
Author information
Authors and Affiliations
Contributions
All authors drafted and reviewed the manuscript, approved the final version, agreed to publish this report, and vouch for accuracy and completeness of these data.
Corresponding author
Ethics declarations
Conflict of interest
SIida received honoraria and research funding from Janssen, Sanofi, Takeda, Ono, Celgene, Bristol Myers Squibb, and Daiichi Sankyo; and received research funding from Chugai, Kyowa Kirin, AbbVie, and Merck Sharp & Dohme. KK received honoraria from Janssen, Celgene, Takeda, and Amgen; and received a research grant from Celgene and Janssen. SZU served as a consultant or advisor for Amgen, Bristol Myers Squibb, Celgene, Janssen, Merck, SkylineDX, and Takeda; received a research grant from Amgen, Array BioPharma, Bristol Myers Squibb, Celgene, Janssen, Merck, Pharmacyclics, Sanofi, and Takeda; and served on speakers bureaus for Amgen, Celgene, Janssen, Sanofi, and Takeda. M-VM served on an advisory committee for AbbVie, Amgen, Celgene, Genentech, GlaxoSmithKline, Janssen, Mundipharma EDO, PharmaMar, Roche, and Takeda; served on data and monitoring committees for Amgen and Janssen; served on speakers bureaus for Amgen, Celgene, Janssen, and Takeda; received honoraria from Janssen, Celgene, Takeda, Amgen, and Adaptive; and was a member of a board of directors or advisory committee for Janssen, Celgene, Takeda, Amgen, GlaxoSmithKline, AbbVie, Mundipharma EDO, and PharmaMar. CH, XQ, DAP, KSG, and MQ are employed by and own equity in Janssen. NB served as a consultant for and received honoraria from Janssen, Celgene, Amgen, Takeda, and AbbVie. SIto received honoraria from Janssen, Celgene, Bristol Myers Squibb, Ono, and Takeda. TI, CKM, SPY, and HN declare that they have no conflicts of interest.
Ethical approval
An independent ethics committee or institutional review board approved the trial. The study protocol was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation guidelines on Good Clinical Practice.
Consent to participate
All patients provided written informed consent.
Consent for publication
Not applicable.
Code availability
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 981 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iida, S., Ishikawa, T., Min, C.K. et al. Subcutaneous daratumumab in Asian patients with heavily pretreated multiple myeloma: subgroup analyses of the noninferiority, phase 3 COLUMBA study. Ann Hematol 100, 1065–1077 (2021). https://doi.org/10.1007/s00277-021-04405-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-021-04405-2