Abstract
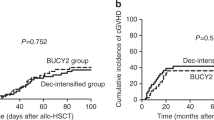
The aim of this study is to determine whether the modified BuCy (semustine, cytarabine, busulfan, and cyclophosphamide, mBuCy) conditioning regimen can be safely used as an alternative to the SEAM (semustine, etoposide, cytarabine, and melphalan) regimen by comparing the efficacy and toxicity of the mBuCy and SEAM regimens. We matched 34 pairs of patients with regard to disease status at the time of autologous stem cell transplantation (auto-SCT). We found no significant difference in the time of platelet engraftment between the two groups. Furthermore, neutrophil engraftment was somewhat faster in the mBuCy group than in the SEAM group (median: 9 days vs 10 days, p = 0.015). With regard to toxicity, the incidence of nausea/vomiting, hepatic impairment, renal impairment, pulmonary infection, and treatment-related mortality (TRM) was similar between the two groups. In addition, compared to patients conditioned with SEAM, patients conditioned with mBuCy were less likely to develop mucositis and diarrhea (p = 0.027; p = 0.050). The 2-year progression-free survival (PFS) rates in the mBuCy and SEAM groups were 79% and 70% (p = 0.378), respectively, and the 2-year overall survival (OS) rates were 81% and 78.0%, respectively (p = 0.789). These analyses showed that the mBuCy conditioning regimen was well tolerated and can be used as an alternative to the SEAM regimen for lymphoma.

Similar content being viewed by others
References
Colpo A, Hochberg E, Chen YB (2012) Current status of autologous stem cell transplantation in relapsed and refractory Hodgkin’s lymphoma. Oncologist 17:80–90
Haioun C, Lepage E, Gisselbrecht C, Bastion Y, Coiffier B, Brice P, Bosly A, Dupriez B, Nouvel C, Tilly H, Lederlin P, Biron P, Brière J, Gaulard P, Reyes F (1997) Benefit of autologous bone marrow transplantation over sequential chemotherapy in poor-risk aggressive non-Hodgkin’s lymphoma: updated results of the prospective study LNH87-2. Groupe d’Etude des Lymphomes de l’Adulte. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 15:1131–1137
Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A et al (1993) Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet 341:1051–1054
Gaspard MH, Maraninchi D, Stoppa AM, Gastaut JA, Michel G, Tubiana N, Blaise D, Novakovitch G, Rossi JF, Weiller PJ, Sainty D, Horchowski N, Carcassonne Y (1988) Intensive chemotherapy with high doses of BCNU, etoposide, cytosine arabinoside, and melphalan (BEAM) followed by autologous bone marrow transplantation: toxicity and antitumor activity in 26 patients with poor-risk malignancies. Cancer Chemother Pharmacol 22:256–262
Mills W, Chopra R, McMillan A, Pearce R, Linch DC, Goldstone AH (1995) BEAM chemotherapy and autologous bone marrow transplantation for patients with relapsed or refractory non-Hodgkin’s lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 13:588–595
Cortelazzo S, Rossi A, Viero P, Bellavita P, Marchioli R, Marfisi RM, Rambaldi A, Barbui T (1997) BEAM chemotherapy and autologous haemopoietic progenitor cell transplantation as front-line therapy for high-risk patients with diffuse large cell lymphoma. Br J Haematol 99:379–385
Cioch M, Jawniak D, Wach M, Manko J, Radomska K, Borowska H et al (2016) Autologous hematopoietic stem cell transplantation for adults with acute myeloid leukemia. Transplant Proc 48:1814–1817
Vellenga E, van Putten W, Ossenkoppele GJ, Verdonck LF, Theobald M, Cornelissen JJ, Huijgens PC, Maertens J, Gratwohl A, Schaafsma R, Schanz U, Graux C, Schouten HC, Ferrant A, Bargetzi M, Fey MF, Lowenberg B, for the Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON), and Swiss Group for Clinical Cancer Research Collaborative Group (SAKK) (2011) Autologous peripheral blood stem cell transplantation for acute myeloid leukemia. Blood 118:6037–6042
Zhang QY, Huang WR, Dou LP, Deng AL, Fu L, Xu YH et al (2014) Effect of autologous peripheral blood stem cell transplantation in 13 patients with AML1/ETO (+) acute myeloid leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi 22:447–452
Chen J, Yang L, Fan Y, Xu Y, Han Y, Tang X et al (2017) Comparison of autologous stem cell transplantation versus haploidentical donor stem cell transplantation for favorable- and intermediate-risk acute myeloid leukemia patients in first complete remission. Biol Blood Marrow Transplant
Burchenal JH, Carter SK (1972) New cancer chemotherapeutic agents. Cancer 30:1639–1646
Oliverio VT (1973) Toxicology and pharmacology of the nitrosoureas. Cancer Chemother Rep 3 4:13–20
Young RC (1973) The effect of methyl CCNU (NSC-95441) on the cellular kinetics of normal and leukemic murine tissues in vivo. Cell Tissue Kinet 6:35–43
Hill DL, Kirk MC, Struck RF (1975) Microsomal metabolism of nitrosoureas. Cancer Res 35:296–301
Sponzo RW, DeVita VT, Oliverio VT (1973) Physiologic disposition of 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) and 1-(2-chloroethyl)-3-(4-methyl cyclohexyl)-1-nitrosourea (Me CCNU) in man. Cancer 31:1154–1156
Wheeler GP (1962) Studies related to the mechanisms of action of cytotoxic alkylating agents: a review. Cancer Res 22:651–688
Redondo AM, Pomares H, Vidal MJ, Pascual MJ, Quereda B, Sancho JM, Polo M, López J, Conde E, Jarque I, Alonso N, Ramírez MJ, Fernández P, Sayas MJ, Requena MJ, Salar A, González JD, González-Barca E, Arranz R, Caballero D, Martín A (2014) Impact of prior rituximab on outcomes of autologous stem-cell transplantation in patients with relapsed or refractory aggressive B-cell lymphoma: a multicentre retrospective Spanish group of lymphoma/autologous bone marrow transplant study. Br J Haematol 164:668–674
Gui L, Shi YK, He XH, Lei YH, Zhang HZ, Han XH, Zhou SY, Liu P, Yang JL, Dong M, Zhang CG, Yang S, Qin Y (2014) High-dose therapy and autologous stem cell transplantation in peripheral T-cell lymphoma: treatment outcome and prognostic factor analysis. Int J Hematol 99:69–78
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-López A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP (1999) Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI sponsored international working group. J Clin Oncol 17:1244
Caballero D, Rubio V, Rifon J, Heras I, Garcia-Sanz R, Vidriales B et al (1997) Autologous transplant with BEAM protocol in lymphoma. Sangre 42(Suppl 1):46–49
Caballero MD, Rubio V, Rifon J, Heras I, Garcia-Sanz R, Vazquez L et al (1997) BEAM chemotherapy followed by autologous stem cell support in lymphoma patients: analysis of efficacy, toxicity and prognostic factors. Bone Marrow Transplant 20:451–458
Berber I, Erkurt MA, Nizam I, Koroglu M, Kaya E, Kuku I, Bag HG (2015) Can BuCyE conditioning regimen be an alternative treatment to BEAM at autologous transplantation in malignant lymphoma patients?: a single center experience. Int J Clin Exp Med 8:16308–16314
Kim JE, Lee DH, Yoo C, Kim S, Kim SW, Lee JS, Park CJ, Huh J, Suh C (2011) BEAM or BuCyE high-dose chemotherapy followed by autologous stem cell transplantation in non-Hodgkin’s lymphoma patients: a single center comparative analysis of efficacy and toxicity. Leuk Res 35:183–187
Samuels BL, Bitran JD (1995) High-dose intravenous melphalan: a review. J Clin Oncol 13:1786–1799
Grochow LB, Jones RJ, Brundrett RB, Braine HG, Chen TL, Saral R, Santos GW, Colvin OM (1989) Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol 25:55–61
Hagglund H, Ringden O, Ericzon BG, Duraj F, Ljungman P, Lonnqvist B et al (1996) Treatment of hepatic venoocclusive disease with recombinant human tissue plasminogen activator or orthotopic liver transplantation after allogeneic bone marrow transplantation. Transplantation 62:1076–1080
Ulrickson M, Aldridge J, Kim HT, Hochberg EP, Hammerman P, Dube C, Attar E, Ballen KK, Dey BR, McAfee SL, Spitzer TR, Chen YB (2009) Busulfan and cyclophosphamide (Bu/Cy) as a preparative regimen for autologous stem cell transplantation in patients with non-Hodgkin lymphoma: a single-institution experience. Biol Blood Marrow Transplant 15:1447–1454
Sharma A, Kayal S, Iqbal S, Malik PS, Raina V (2013) Comparison of BEAM vs. LEAM regimen in autologous transplant for lymphoma at AIIMS. Springerplus 2:489
Wadehra N, Farag S, Bolwell B, Elder P, Penza S, Kalaycio M, Avalos B, Pohlman B, Marcucci G, Sobecks R, Lin T, Andrèsen S, Copelan E (2006) Long-term outcome of Hodgkin disease patients following high-dose busulfan, etoposide, cyclophosphamide, and autologous stem cell transplantation. Biol Blood Marrow Transplant 12:1343–1349
Funding
This work was supported by grants from the Six Talent Peaks Project in Jiangsu Province (WSN 020).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This study was conducted in compliance with the institutional policy regarding the protection of patients’ private information and approved by the Research Ethics Committee of the First Affiliated Hospital of Soochow University. All the methods were carried out in accordance with the approval guidelines of The First Affiliated Hospital of Soochow University.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Haiwen Huang and Lihong Zhang are co-first authors.
Rights and permissions
About this article
Cite this article
Huang, H., Zhang, L., Jiang, Y. et al. Modified BuCy is an alternative conditioning regimen for lymphoma patients undergoing autologous stem cell transplantation. Ann Hematol 98, 1259–1266 (2019). https://doi.org/10.1007/s00277-018-3576-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-018-3576-2