Abstract
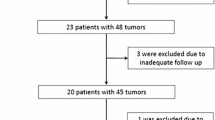
The purpose of this study was to investigate differences in change of size and CT value between local recurrences and tumor-free areas after CT-guided radiofrequency ablation (RFA) of hepatic metastases during follow-up by means of dedicated software for automatic evaluation of hepatic lesions. Thirty-two patients with 54 liver metastases from breast or colorectal cancer underwent triphasic contrast-enhanced multidetector-row computed tomography (MDCT) to evaluate hepatic metastatic spread and localization before CT-guided RFA and for follow-up after intervention. Sixteen of these patients (65.1 ± 10.3 years) with 30 metastases stayed tumor-free (group 1), while the other group (n = 16 with 24 metastases; 62.0 ± 13.8 years) suffered from local recurrent disease (group 2). Applying an automated software tool (SyngoCT Oncology; Siemens Healthcare, Forchheim, Germany), size parameters (volume, RECIST, WHO) and attenuation were measured within the lesions before, 1 day after, and 28 days after RFA treatment. The natural logarithm (ln) of the quotient of the volume 1 day versus 28 days after RFA treament was computed: lnQ1//28/0volume. Analogously, ln ratios of RECIST, WHO, and attenuation were computed and statistically evaluated by repeated-measures ANOVA. One lesion in group 2 was excluded from further evaluation due to automated missegmentation. Statistically significant differences between the two groups were observed with respect to initial volume, RECIST, and WHO (p < 0.05). Furthermore, ln ratios corresponding to volume, RECIST, and WHO differed significantly between the two groups. Attenuation evaluations showed no significant differences, but there was a trend toward attenuation assessment for the parameter lnQ28/0attenuation (p = 0.0527), showing higher values for group 1 (–0.4 ± 0.3) compared to group 2 (–0.2 ± 0.2). In conclusion, hepatic metastases and their zone of coagulation necrosis after RFA differed significantly between tumor-free and local-recurrent ablation zones with respect to the corresponding size parameters. A new parameter (lnQ1//28/0volume/RECIST/WHO/attenuation) was introduced, which appears to be of prognostic value at early follow-up CT.





Similar content being viewed by others
References
Mergo PJ, Ros PR (1998) Imaging of diffuse liver disease. Radiol Clin North Am 36:365–375
Boyle P, Ferlay J (2005) Cancer incidence and mortality in Europe, 2004. Ann Oncol 16:481–488
Landis SH, Murray T, Bolden S et al (1999) Cancer statistics, 1999. CA Cancer J Clin 49:8–31, 1
Greenlee RT, Hill-Harmon MB, Murray T et al (2001) Cancer statistics, 2001. CA Cancer J Clin 51:15–36
Hoe AL, Royle GT, Taylor I (1991) Breast liver metastases—incidence, diagnosis and outcome. J R Soc Med 84:714–716
McGhana JP, Dodd GD III (2001) Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol 176:3–16
Rhim H, Lim HK, Kim YS et al (2008) Radiofrequency ablation of hepatic tumors: lessons learned from 3000 procedures. J Gastroenterol Hepatol 23:1492–1500
Buscarini E, Buscarini L (2004) Radiofrequency thermal ablation with expandable needle of focal liver malignancies: complication report. Eur Radiol 14:31–37
Mulier S, Ni Y, Miao Y et al (2003) Size and geometry of hepatic radiofrequency lesions. Eur J Surg Oncol 29(10):867–878
Kim YS, Rhim H, Cho OK et al (2006) Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: analysis of the pattern and risk factors. Eur J Radiol 59:432–441
Mahnken AH, Bruners P, Tacke JA et al (2009) CT-guided radiofrequency ablation of liver metastases from colorectal cancer. Dtsch Med Wochenschr 134:976–980
Abdalla EK, Vauthey JN, Ellis LM et al (2004) Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 239:818–825; discussion 825–827
Bruners P, Pfeffer J, Kazim RM et al (2007) A newly developed perfused umbrella electrode for radiofrequency ablation: an ex vivo evaluation study in bovine liver. Cardiovasc Interv Radiol 30:992–998
Veltri A, Sacchetto P, Tosetti I et al (2008) Radiofrequency ablation of colorectal liver metastases: small size favorably predicts technique effectiveness and survival. Cardiovasc Interv Radiol 31(5):948–956
Dromain C, de Baere T, Elias D et al (2002) Hepatic tumors treated with percutaneous radio-frequency ablation: CT and MR imaging follow-up. Radiology 223:255–262
Wormanns D, Kohl G, Klotz E et al (2004) Volumetric measurements of pulmonary nodules at multi-row detector CT: in vivo reproducibility. Eur Radiol 14:86–92
Marten K, Auer F, Schmidt S et al (2006) Inadequacy of manual measurements compared to automated CT volumetry in assessment of treatment response of pulmonary metastases using RECIST criteria. Eur Radiol 16:781–790
Fabel M, von Tengg-Kobligk H, Giesel FL et al (2008) Semi-automated volumetric analysis of lymph node metastases in patients with malignant melanoma stage III/IV—a feasibility study. Eur Radiol 18:1114–1122
Kuhnigk JM, Dicken V, Bornemann L et al (2006) Morphological segmentation and partial volume analysis for volumetry of solid pulmonary lesions in thoracic CT scans. IEEE Trans Med Imaging 25:417–434
Bornemann L, Dicken V, Kuhnigk JM et al (2007) OncoTREAT: a software assistant for cancer therapy monitoring. Int J Comput Assist Radiol Surg 1:231–242
Benson AB III (2007) Epidemiology, disease progression, and economic burden of colorectal cancer. J Manage Care Pharm 13:5–18
Pereira PL (2007) Actual role of radiofrequency ablation of liver metastases. Eur Radiol 17:2062–2070
Jakobs TF, Hoffmann RT, Schrader A et al (2008) CT-guided radiofrequency ablation in patients with hepatic metastases from breast cancer. Cardiovasc Interv Radiol [Epub ahead of print]
Park MH, Rhim H, Kim YS et al (2008) Spectrum of CT findings after radiofrequency ablation of hepatic tumors. Radiographics 28:379–390; discussion 390–392
Goldberg SN, Grassi CJ, Cardella JF et al (2005) Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology 235:728–739
Mulier S, Ni Y, Jamart J et al (2005) Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg 242:158–171
Paulet E, Aube C, Pessaux P et al (2008) Factors limiting complete tumor ablation by radiofrequency ablation. Cardiovasc Intervent Radiol 31:107–115
Kühl H, Stattaus J, Kühl B et al (2006) Radiofrequency ablation of malignant liver tumors: use of a volumetric necrosis-tumor ratio for local control. Fortschr Roentgenstr 178:1243–1249 [in German]
Prasad SR, Jhaveri KS, Saini S et al (2002) CT tumor measurement for therapeutic response assessment: comparison of unidimensional, bidimensional, and volumetric techniques initial observations. Radiology 225:416–419
Keil S, Behrendt FF, Stanzel S et al (2009) Semi-automated measurement of hyperdense, hypodense and heterogeneous hepatic metastasis on standard MDCT slices. Comparison of semi-automated and manual measurement of RECIST and WHO criteria. Eur Radiol 18:2456–2465
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keil, S., Bruners, P., Schiffl, K. et al. Radiofrequency Ablation of Liver Metastases—Software-Assisted Evaluation of the Ablation Zone in MDCT: Tumor-Free Follow-Up Versus Local Recurrent Disease. Cardiovasc Intervent Radiol 33, 297–306 (2010). https://doi.org/10.1007/s00270-009-9681-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-009-9681-9