Abstract
Purpose
To evalute the efficacy and safety of two low-dose peri-operative dexamethasone on pain and recovery following total hip arthroplasty (THA).
Methods
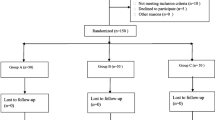
One hundred ten patients received two-dose of 10 mg IV-dexamethasone (group dexa) or IV-isotonic saline (group placebo). The level of C-reactive protein (CRP) and interleukin-6 (IL-6), pain at rest and during mobilization, incidence of post-operative nausea and vomiting (PONV), intensity of nausea, post-operative fatigue, consumption of analgesic and antiemetic rescue, range of motion (ROM), post-operative length of stay (post-operative LOS), wound problems and complications were recorded and compared.
Results
The level of inflammation markers (CRP, IL-6) in group dexa was lower than group placebo at 24, 48, 72 hours post-operatively. Dynamic pain VAS score at 24 hours was lower in group dexa (P = 0.002), however, there was no significant effect on pain at rest. In group dexa, patients had a lower incidence of PONV (P = 0.003), as well as a lower VAS score of nausea (P = 0.044). The post-operative fatigue (P < 0.001) was relieved and the consumption of analgesic and antiemetic rescues were reduced. Furthermore, patients had better maximum hip flexion (P < 0.001) and abduction (P = 0.017), with shorter post-operative LOS (P = 0.006). There is no difference between groups in wound problems. No surgical site infection or gastrointestinal haemorrhage was detected in both groups.
Conclusions
The administration of two low-dose peri-operative dexamethasone can effectively reduce the post-operative level of CRP and IL-6, provide additional pain and nausea control, ameliorate post-operative fatigue, enhance mobility, and shorten post-operative LOS following THA, without increasing the risk of infection and gastrointestinal hemorrhage.
Level of evidence: I



Similar content being viewed by others
References
Yue C, Kang P, Yang P, Xie J, Pei F (2014) Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplast 29(12):2452–2456. doi:10.1016/j.arth.2014.03.032
Wasko MK, Bobecka-Wesolowska K, Tomasiuk R, Kowalczewski J (2015) Measurement of the inflammatory response in the early postoperative period after hip and knee arthroplasty. Clin Chem Lab Med 53(11):1785–1792. doi:10.1515/cclm-2014-1055
Aasvang EK, Luna IE, Kehlet H (2015) Challenges in postdischarge function and recovery: the case of fast-track hip and knee arthroplasty. Br J Anaesth 115(6):861–866. doi:10.1093/bja/aev257
Richardson AB, Bala A, Wellman SS, Attarian DE, Bolognesi MP, Grant SA (2016) Perioperative dexamethasone administration does not increase the incidence of postoperative infection in Total hip and knee Arthroplasty: a retrospective analysis. J Arthroplast 31(8):1784–1787. doi:10.1016/j.arth.2016.01.028
Bolac CS, Wallace AH, Broadwater G, Havrilesky LJ, Habib AS (2013) The impact of postoperative nausea and vomiting prophylaxis with dexamethasone on postoperative wound complications in patients undergoing laparotomy for endometrial cancer. Anesth Analg 116(5):1041–1047. doi:10.1213/ANE.0b013e318276cf58
Chen CC, Siddiqui FJ, Chen TL, Chan ES, Tam KW (2012) Dexamethasone for prevention of postoperative nausea and vomiting in patients undergoing thyroidectomy: meta-analysis of randomized controlled trials. World J Surg 36(1):61–68. doi:10.1007/s00268-011-1343-9
Romundstad L, Breivik H, Roald H, Skolleborg K, Haugen T, Narum J, Stubhaug A (2006) Methylprednisolone reduces pain, emesis, and fatigue after breast augmentation surgery: a single-dose, randomized, parallel-group study with methylprednisolone 125 mg, parecoxib 40 mg, and placebo. Anesth Analg 102(2):418–425. doi:10.1213/01.ane.0000194358.46119.e1
Lunn TH, Andersen LO, Kristensen BB, Husted H, Gaarn-Larsen L, Bandholm T, Ladelund S, Kehlet H (2013) Effect of high-dose preoperative methylprednisolone on recovery after total hip arthroplasty: a randomized, double-blind, placebo-controlled trial. Br J Anaesth 110(1):66–73. doi:10.1093/bja/aes345
Backes JR, Bentley JC, Politi JR, Chambers BT (2013) Dexamethasone reduces length of hospitalization and improves postoperative pain and nausea after total joint arthroplasty: a prospective, randomized controlled trial. J Arthroplast 28(8 Suppl):11–17. doi:10.1016/j.arth.2013.05.041
Miyagawa Y, Ejiri M, Kuzuya T, Osada T, Ishiguro N, Yamada K (2010) Methylprednisolone reduces postoperative nausea in total knee and hip arthroplasty. J Clin Pharm Ther 35(6):679–684. doi:10.1111/j.1365-2710.2009.01141.x
Romundstad L, Breivik H, Niemi G, Helle A, Stubhaug A (2004) Methylprednisolone intravenously 1 day after surgery has sustained analgesic and opioid-sparing effects. Acta Anaesthesiol Scand 48(10):1223–1231. doi:10.1111/j.1399-6576.2004.00480.x
Lunn TH, Kehlet H (2013) Perioperative glucocorticoids in hip and knee surgery - benefit vs. harm? A review of randomized clinical trials. Acta Anaesthesiol Scand 57(7):823–834. doi:10.1111/aas.12115
De Oliveira GS Jr, Almeida MD, Benzon HT, McCarthy RJ (2011) Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology 115(3):575–588. doi:10.1097/ALN.0b013e31822a24c2
Kardash KJ, Sarrazin F, Tessler MJ, Velly AM (2008) Single-dose dexamethasone reduces dynamic pain after total hip arthroplasty. Anesth Analg 106(4):1253–1257, table of contents. doi:10.1213/ANE.0b013e318164f319
Bergeron SG, Kardash KJ, Huk OL, Zukor DJ, Antoniou J (2009) Perioperative dexamethasone does not affect functional outcome in total hip arthroplasty. Clin Orthop Relat Res 467(6):1463–1467. doi:10.1007/s11999-009-0733-x
Cui Z, Liu X, Teng Y, Jiang J, Wang J, Xia Y (2015) The efficacy of steroid injection in total knee or hip arthroplasty. Knee Surg Sports Traumatol Arthrosc 23(8):2306–2314. doi:10.1007/s00167-014-3049-7
Spies CM, Strehl C, van der Goes MC, Bijlsma JW, Buttgereit F (2011) Glucocorticoids. Best Pract Res Clin Rheumatol 25(6):891–900. doi:10.1016/j.berh.2011.11.002
Coutinho AE, Chapman KE (2011) The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol 335(1):2–13. doi:10.1016/j.mce.2010.04.005
Louati K, Berenbaum F (2015) Fatigue in chronic inflammation — a link to pain pathways. Arthritis Res Ther 17:254. doi:10.1186/s13075-015-0784-1
Sculco PK, McLawhorn AS, Desai N, Su EP, Padgett DE, Jules-Elysee K (2016) The effect of perioperative corticosteroids in Total hip Arthroplasty: a prospective double-blind placebo controlled pilot study. J Arthroplast 31(6):1208–1212. doi:10.1016/j.arth.2015.11.011
Mathiesen O, Jacobsen LS, Holm HE, Randall S, Adamiec-Malmstroem L, Graungaard BK, Holst PE, Hilsted KL, Dahl JB (2008) Pregabalin and dexamethasone for postoperative pain control: a randomized controlled study in hip arthroplasty. Br J Anaesth 101(4):535–541. doi:10.1093/bja/aen215
Nostdahl T, Bernklev T, Raeder J, Sandvik L, Fredheim O (2016) Postoperative fatigue; translation and validation of a revised 10-item short form of the Identity-Consequence fatigue scale (ICFS). J Psychosom Res 84:1–7. doi:10.1016/j.jpsychores.2016.03.002
Rubin GJ, Hotopf M (2002) Systematic review and meta-analysis of interventions for postoperative fatigue. Br J Surg 89(8):971–984. doi:10.1046/j.1365-2168.2002.02138.x
Sibia US, MacDonald JH, King PJ (2016) Predictors of hospital length of stay in an enhanced recovery after surgery program for primary total hip arthroplasty. J Arthroplast 31(10):2119–2123. doi:10.1016/j.arth.2016.02.060
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Funding
This study was funded by the National Health and Family Planning Commission of the People’s Republic of China (CN) program (201302007).
Ethical approval
The trial was approved by the institutional review board and registered in the International Clinical Trial Registry (ChiCTR-IOR-16008865).
Informed consent
Informed consent was obtained from all participants included in the study.
Rights and permissions
About this article
Cite this article
Lei, Yt., Xu, B., Xie, Xw. et al. The efficacy and safety of two low-dose peri-operative dexamethasone on pain and recovery following total hip arthroplasty: a randomized controlled trial. International Orthopaedics (SICOT) 42, 499–505 (2018). https://doi.org/10.1007/s00264-017-3537-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-017-3537-8