Abstract
Purpose
The purpose of this study is to assess safety and feasibility of intradiscal bone marrow concentrate (BMC) injections to treat discogenic pain as an alternative to surgery.
Methods
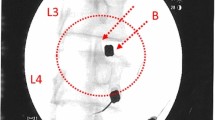
A total of 26 patients (11 male, 15 female, aged 18–61 years, 13 single level, 13 two level) that met inclusion criteria of chronic (> 6 months) discogenic low back pain, degenerative disc pathology assessed by magnetic resonance imaging (MRI) with modified Pfirrmann grade of IV–VII at one or two levels, candidate for surgical intervention (failed conservative treatment and radiologic findings) and a visual analogue scale (VAS) pain score of 40 mm or more at initial visit. Initial Oswestry Disability Index (ODI) and VAS pain score average was 56.5 % and 80.1 mm (0–100), respectively. Adverse event reporting, ODI score, VAS pain score, MRI radiographic changes, progression to surgery and cellular analysis of BMC were noted. Retrospective cell analysis by flow cytometry and colony forming unit-fibroblast (CFU-F) assays were performed to characterise each patient’s BMC and compare with clinical outcomes. The BMC was injected into the nucleus pulposus of the symptomatic disc(s) under fluoroscopic guidance. Patients were evaluated clinically prior to treatment and at three, six, 12 and 24 months and radiographically prior to treatment and at 12 months.
Results
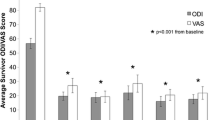
There were no complications from the percutaneous bone marrow aspiration or disc injection. Of 26 patients, 24 (92 %) avoided surgery through 12 months, while 21 (81 %) avoided surgery through two years. Of the 21 surviving patients, the average ODI and VAS scores were reduced to 19.9 and 27.0 at three months and sustained to 18.3 and 22.9 at 24 months, respectively (p ≤ 0.001). Twenty patients had follow-up MRI at 12 months, of whom eight had improved by at least one Pfirrmann grade, while none of the discs worsened. Total and rate of pain reduction were linked to mesenchymal stem cell concentration through 12 months. Only five of the 26 patients elected to undergo surgical intervention (fusion or artificial disc replacement) by the two year milestone.
Conclusions
This study provides evidence of safety and feasibility in the non-surgical treatment of discogenic pain with autologous BMC, with durable pain relief (71 % VAS reduction) and ODI improvements (> 64 %) through two years.


Similar content being viewed by others
References
Dagenais S, Caro J, Haldeman S (2008) A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J 8:8–20. doi:10.1016/j.spinee.2007.10.005
Luo X, Pietrobon R, Sun SX et al (2004) Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine 29:79–86. doi:10.1097/01.BRS.0000105527.13866.0F
Schnitzer TJ, Ferraro A, Hunsche E, Kong SX (2004) A comprehensive review of clinical trials on the efficacy and safety of drugs for the treatment of low back pain. J Pain Symptom Manage 28:72–95. doi:10.1016/j.jpainsymman.2003.10.015
White AP, Arnold PM, Norvell DC et al (2011) Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine 36:S131–S143. doi:10.1097/BRS.0b013e31822f178f
Gornet MF, Burkus JK, Dryer RF, Peloza JH (2011) Lumbar disc arthroplasty with Maverick disc versus stand-alone interbody fusion: a prospective, randomized, controlled, multicenter investigational device exemption trial. Spine 36:E1600–E1611. doi:10.1097/BRS.0b013e318217668f
Zigler J, Delamarter R, Spivak JM et al (2007) Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine 32:1155–1162. doi:10.1097/BRS.0b013e318054e377, discussion 1163
Phillips FM, Slosar PJ, Youssef JA et al (2013) Lumbar spine fusion for chronic low back pain due to degenerative disc disease: a systematic review. Spine (Phila Pa 1976) 38:E409–E422. doi:10.1097/BRS.0b013e3182877f11
Deyo RA, Gray DT, Kreuter W et al (2005) United States trends in lumbar fusion surgery for degenerative conditions. Spine 30:1441–1445. doi:10.1097/01.brs.0000166503.37969.8a, discussion 1446–1447
Cheng JS, Lee MJ, Massicotte E et al (2011) Clinical guidelines and payer policies on fusion for the treatment of chronic low back pain. Spine 36:S144–S163. doi:10.1097/BRS.0b013e31822ef5b4
Bederman SS (2012) Commentary: the degenerative lumbar spine: a chronic condition in search of a definitive solution. Spine J 12:98–100. doi:10.1016/j.spinee.2012.01.005
Murphy MB, Moncivais K, Caplan AI (2013) Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 45:e54. doi:10.1038/emm.2013.94
Buchanan RM, Blashki D, Murphy MB (2014) Stem cell therapy for regenerative medicine. Chem Eng Prog 110:55–58
Hernigou P, Homma Y, Flouzat Lachaniette HC et al (2013) Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int Orthop 37:2279–2287. doi: 10.1007/s00264-013-2017-z
Mehling WE, Gopisetty V, Bartmess E et al (2012) The prognosis of acute low back pain in primary care in the United States: a 2-year prospective cohort study. Spine 37:678–684. doi:10.1097/BRS.0b013e318230ab20
Weber H, Holme I, Amlie E (1993) The natural course of acute sciatica with nerve root symptoms in a double-blind placebo-controlled trial evaluating the effect of piroxicam. Spine 18:1433–1438
Carey TS, Garrett J, Jackman A et al (1995) The outcomes and costs of care for acute low back pain among patients seen by primary care practitioners, chiropractors, and orthopedic surgeons. The North Carolina Back Pain Project. N Engl J Med 333:913–917. doi:10.1056/NEJM199510053331406
Steele J, Bruce-Low S, Smith D et al (2013) A randomized controlled trial of limited range of motion lumbar extension exercise in chronic low back pain. Spine 38:1245–1252. doi:10.1097/BRS.0b013e318291b526
Becker A, Held H, Redaelli M et al (2012) Implementation of a guideline for low back pain management in primary care: a cost-effectiveness analysis. Spine 37:701–710. doi:10.1097/BRS.0b013e31822b01bd
Soer R, Reneman MF, Vroomen PCAJ et al (2012) Responsiveness and minimal clinically important change of the Pain Disability Index in patients with chronic back pain. Spine 37:711–715. doi:10.1097/BRS.0b013e31822c8a7a
Gillard DM, Corenman DS, Dornan GJ (2014) Failed less invasive lumbar spine surgery as a predictor of subsequent fusion outcomes. Int Orthop 38:811–815. doi:10.1007/s00264-013-2167-z
Kanayama M, Oha F, Hashimoto T (2015) What types of degenerative lumbar pathologies respond to nerve root injection? A retrospective review of six hundred and forty one cases. Int Orthop 39:1379–1382. doi:10.1007/s00264-015-2761-3
Brox JI, Nygaard ØP, Holm I et al (2010) Four-year follow-up of surgical versus non-surgical therapy for chronic low back pain. Ann Rheum Dis 69:1643–1648. doi:10.1136/ard.2009.108902
Fairbank J, Frost H, Wilson-MacDonald J et al (2005) Randomised controlled trial to compare surgical stabilisation of the lumbar spine with an intensive rehabilitation programme for patients with chronic low back pain: the MRC spine stabilisation trial. BMJ 330:1233. doi:10.1136/bmj.38441.620417.8F
Fritzell P, Hägg O, Wessberg P, Nordwall A et al (2001) 2001 Volvo Award winner in Clinical Studies: lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine 26:2521–2532, discussion 2532–2534
Ohtori S, Koshi T, Yamashita M et al (2011) Surgical versus nonsurgical treatment of selected patients with discogenic low back pain: a small-sized randomized trial. Spine 36:347–354. doi:10.1097/BRS.0b013e3181d0c944
Coric D, Pettine KA, Sumich A, Boltes MO (2013) Prospective study of disc repair with allogeneic chondrocytes presented at the 2012 Joint Spine Section Meeting. J Neurosurg Spine 18:85–95. doi: 10.3171/2012.10.SPINE12512
Murphy MB, Blashki D, Buchanan RM et al (2012) Adult and umbilical cord blood-derived platelet-rich plasma for mesenchymal stem cell proliferation, chemotaxis, and cryo-preservation. Biomaterials 33:5308–5316. doi:10.1016/j.biomaterials.2012.04.007
Pettine KA, Murphy MB, Suzuki RK, Sand TT (2015) Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells 33:146–156. doi:10.1002/stem.1845
Fischer CA, Neubauer E, Adams HS et al (2014) Effects of multidisciplinary pain treatment can be predicted without elaborate questionnaires. Int Orthop 38:617–626. doi:10.1007/s00264-013-2156-2
Acknowledgments
The authors thank Mr. Tyler Santomaso and Ms. Melissa Samano for their contributions in data collection and review. Celling Biosciences (Austin, TX) provided concentration devices for the processing of each patient’s bone marrow aspirate.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pettine, K., Suzuki, R., Sand, T. et al. Treatment of discogenic back pain with autologous bone marrow concentrate injection with minimum two year follow-up. International Orthopaedics (SICOT) 40, 135–140 (2016). https://doi.org/10.1007/s00264-015-2886-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-015-2886-4