Abstract
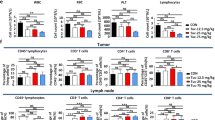
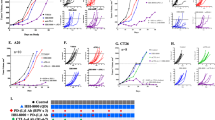
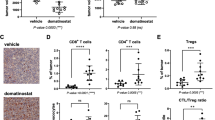
Checkpoint inhibitor therapy has led to major treatment advances for several cancers including non-small cell lung cancer (NSCLC). Despite this, a significant percentage of patients do not respond or develop resistance. Potential mechanisms of resistance include lack of expression of programmed death ligand 1 (PD-L1), decreased capacity to present tumor antigens, and the presence of an immunosuppressive tumor microenvironment. Mocetinostat is a spectrum-selective inhibitor of class I/IV histone deacetylases (HDACs), a family of proteins implicated in epigenetic silencing of immune regulatory genes in tumor and immune cells. Mocetinostat upregulated PD-L1 and antigen presentation genes including class I and II human leukocyte antigen (HLA) family members in a panel of NSCLC cell lines in vitro. Mocetinostat target gene promoters were occupied by a class I HDAC and exhibited increased active histone marks after mocetinostat treatment. Mocetinostat synergized with interferon γ (IFN-γ) in regulating class II transactivator (CIITA), a master regulator of class II HLA gene expression. In a syngeneic tumor model, mocetinostat decreased intratumoral T-regulatory cells (Tregs) and potentially myeloid-derived suppressor cell (MDSC) populations and increased intratumoral CD8+ populations. In ex vivo assays, patient-derived, mocetinostat-treated Tregs also showed significant down regulation of FOXP3 and HELIOS. The combination of mocetinostat and a murine PD-L1 antibody antagonist demonstrated increased anti-tumor activity compared to either therapy alone in two syngeneic tumor models. Together, these data provide evidence that mocetinostat modulates immune-related genes in tumor cells as well as immune cell types in the tumor microenvironment and enhances checkpoint inhibitor therapy.






Similar content being viewed by others
Abbreviations
- CIITA:
-
Class II transactivator
- Ccl5:
-
Chemokine (C–C motif) ligand 5
- CDKN1A:
-
Cyclin-dependent kinase inhibitor 1A
- CDR3:
-
Complementarity determining region 3
- ChIP-Seq:
-
Chromatin immunoprecipitation-sequencing
- Cxcr6:
-
Chemokine (C–X–C motif) receptor 6
- FOS:
-
FBJ murine osteosarcoma viral oncogene homolog
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GUSB:
-
Glucuronidase, beta
- H2-Aa:
-
Histocompatibility 2, class II antigen A, alpha
- H3K4me3:
-
Histone 3 lysine 4 trimethylation
- H3K27Ac:
-
Histone 3 lysine 27 acetylation
- HDAC:
-
Histone deacetylase
- HLA:
-
Human leukocyte antigen
- IFN-γ:
-
Interferon γ
- Iso Ab:
-
Isotype antibody
- MDSC:
-
Myeloid-derived suppressor cell
- MHC:
-
Major histocompatibility
- MIC-A/B:
-
MHC class I polypeptide-related sequence A/B
- NSCLC:
-
Non-small cell lung cancer
- RT:
-
Reverse transcription
- SEM:
-
Standard error of the mean
- TCR:
-
T-cell receptor
- Treg:
-
T-regulatory cell
References
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373(17):1627–1639
Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E et al (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373(2):123–135
Callahan MK, Postow MA, Wolchok JD (2016) Targeting T cell co-receptors for cancer therapy. Immunity 44(5):1069–1078
Rizvi NA, Mazieres J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ et al (2015) Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 16(3):257–265
Garraway LA, Lander ES (2013) Lessons from the cancer genome. Cell 153(1):17–37
Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR et al (2014) Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 505(7484):495–501
Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S et al (2016) Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 375(9):819–829
Christiansen AJ, West A, Banks KM, Haynes NM, Teng MW, Smyth MJ et al (2011) Eradication of solid tumors using histone deacetylase inhibitors combined with immune-stimulating antibodies. Proc Natl Acad Sci USA 108(10):4141–4146
Shen L, Ciesielski M, Ramakrishnan S, Miles KM, Ellis L, Sotomayor P et al (2012) Class I histone deacetylase inhibitor entinostat suppresses regulatory T cells and enhances immunotherapies in renal and prostate cancer models. PLoS ONE 7(1):e30815
Woods DM, Sodre AL, Villagra A, Sarnaik A, Sotomayor EM, Weber J (2015) HDAC inhibition upregulates PD-1 ligands in melanoma and augments immunotherapy with PD-1 blockade. Cancer Immunol Res 3(12):1375–1385
Guerriero JL, Sotayo A, Ponichtera HE, Castrillon JA, Pourzia AL, Schad S et al (2017) Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature 543(7645):428–432
Kroesen M, Bull C, Gielen PR, Brok IC, Armandari I, Wassink M et al (2016) Anti-GD2 mAb and Vorinostat synergize in the treatment of neuroblastoma. Oncoimmunology 5(6):e1164919
Baylin SB, Jones PA (2016) Epigenetic determinants of cancer. Cold Spring Harb Perspect Biol 8(9):a019505
Kouzarides T (2007) Chromatin modifications and their function. Cell 128(4):693–705
Chen DS, Mellman I (2013) Oncology meets immunology: the cancer-immunity cycle. Immunity 39(1):1–10
Mellman I, Coukos G, Dranoff G (2011) Cancer immunotherapy comes of age. Nature 480(7378):480–489
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4):252–264
Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ et al (2015) Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348(6230):124–128
Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A et al (2014) Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 371(23):2189–2199
Bubenik J (2005) MHC class I down regulation, tumour escape from immune surveillance and design of therapeutic strategies. Folia Biol (Praha) 51(1):1–2
Garcia-Lora A, Algarra I, Garrido F (2003) MHC class I antigens, immune surveillance, and tumor immune escape. J Cell Physiol 195(3):346–355
Maeda T, Towatari M, Kosugi H, Saito H (2000) Up-regulation of costimulatory/adhesion molecules by histone deacetylase inhibitors in acute myeloid leukemia cells. Blood 96(12):3847–3856
Magner WJ, Kazim AL, Stewart C, Romano MA, Catalano G, Grande C et al (2000) Activation of MHC class I, II, and CD40 gene expression by histone deacetylase inhibitors. J Immunol 165(12):7017–7024
Skov S, Pedersen MT, Andresen L, Straten PT, Woetmann A, Odum N (2005) Cancer cells become susceptible to natural killer cell killing after exposure to histone deacetylase inhibitors due to glycogen synthase kinase-3-dependent expression of MHC class I-related chain A and B. Cancer Res 65(23):11136–11145
Woods DM, Woan K, Cheng F, Wang H, Perez-Villarroel P, Lee C et al (2013) The antimelanoma activity of the histone deacetylase inhibitor panobinostat (LBH589) is mediated by direct tumor cytotoxicity and increased tumor immunogenicity. Melanoma Res 23(5):341–348
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS et al (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515(7528):563–567
Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C et al (2014) MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515(7528):558–562
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366(26):2443–2454
Zou W, Wolchok JD, Chen L (2016) PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med 8(328):328rv4
Vanneman M, Dranoff G (2012) Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 12(4):237–251
Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL et al (2014) Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci USA 111(32):11774–11779
Zheng H, Zhao W, Yan C, Watson CC, Massengill M, Xie M et al (2016) HDAC inhibitors enhance T-cell chemokine expression and augment response to PD-1 immunotherapy in lung adenocarcinoma. Clin Cancer Res 22(16):4119–4132
Beier UH, Wang L, Han R, Akimova T, Liu Y, Hancock WW (2012) Histone deacetylases 6 and 9 and sirtuin-1 control Foxp3+ regulatory T cell function through shared and isoform-specific mechanisms. Sci Signal 5(229):ra45
Kalin JH, Butler KV, Akimova T, Hancock WW, Kozikowski AP (2012) Second-generation histone deacetylase 6 inhibitors enhance the immunosuppressive effects of Foxp3+ T-regulatory cells. J Med Chem 55(2):639–651
de Zoeten EF, Wang L, Butler K, Beier UH, Akimova T, Sai H et al (2011) Histone deacetylase 6 and heat shock protein 90 control the functions of Foxp3(+) T-regulatory cells. Mol Cell Biol 31(10):2066–2078
Segretti MC, Vallerini GP, Brochier C, Langley B, Wang L, Hancock WW et al (2015) Thiol-based potent and selective HDAC6 inhibitors promote tubulin acetylation and T-regulatory cell suppressive function. ACS Med Chem Lett 6(11):1156–1161
Youn JI, Kumar V, Collazo M, Nefedova Y, Condamine T, Cheng P et al (2013) Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol 14(3):211–220
Orillion A, Hashimoto A, Damayanti N, Shen L, Adelaiye-Ogala R, Arisa S et al (2017) Entinostat neutralizes myeloid-derived suppressor cells and enhances the antitumor effect of PD-1 inhibition in murine models of lung and renal cell carcinoma. Clin Cancer Res 23(17):5187–5201
Egan B, Yuan CC, Craske ML, Labhart P, Guler GD, Arnott D et al (2016) An alternative approach to ChIP-Seq normalization enables detection of genome-wide changes in histone H3 lysine 27 trimethylation upon EZH2 inhibition. PLoS One 11(11):e0166438
Carlson CS, Emerson RO, Sherwood AM, Desmarais C, Chung MW, Parsons JM et al (2013) Using synthetic templates to design an unbiased multiplex PCR assay. Nat Commun 4:2680
Robins H, Desmarais C, Matthis J, Livingston R, Andriesen J, Reijonen H et al (2012) Ultra-sensitive detection of rare T cell clones. J Immunol Methods 375(1–2):14–19
Robins HS, Campregher PV, Srivastava SK, Wacher A, Turtle CJ, Kahsai O et al (2009) Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood 114(19):4099–4107
Kirsch I, Vignali M, Robins H (2015) T-cell receptor profiling in cancer. Mol Oncol 9(10):2063–2070
Chou SD, Khan AN, Magner WJ, Tomasi TB (2005) Histone acetylation regulates the cell type specific CIITA promoters, MHC class II expression and antigen presentation in tumor cells. Int Immunol 17(11):1483–1494
Khan AN, Gregorie CJ, Tomasi TB (2008) Histone deacetylase inhibitors induce TAP, LMP, Tapasin genes and MHC class I antigen presentation by melanoma cells. Cancer Immunol Immunother 57(5):647–654
Beresford GW, Boss JM (2001) CIITA coordinates multiple histone acetylation modifications at the HLA-DRA promoter. Nat Immunol 2(7):652–657
Masternak K, Peyraud N, Krawczyk M, Barras E, Reith W (2003) Chromatin remodeling and extragenic transcription at the MHC class II locus control region. Nat Immunol 4(2):132–137
Wright KL, Ting JP (2006) Epigenetic regulation of MHC-II and CIITA genes. Trends Immunol 27(9):405–412
Kong X, Fang M, Li P, Fang F, Xu Y (2009) HDAC2 deacetylates class II transactivator and suppresses its activity in macrophages and smooth muscle cells. J Mol Cell Cardiol 46(3):292–299
Masternak K, Reith W (2002) Promoter-specific functions of CIITA and the MHC class II enhanceosome in transcriptional activation. EMBO J 21(6):1379–1388
Niesen MI, Blanck G (2009) Rescue of major histocompatibility-DR surface expression in retinoblastoma-defective, non-small cell lung carcinoma cells by the MS-275 histone deacetylase inhibitor. Biol Pharm Bull 32(3):480–482
Spilianakis C, Papamatheakis J, Kretsovali A (2000) Acetylation by PCAF enhances CIITA nuclear accumulation and transactivation of major histocompatibility complex class II genes. Mol Cell Biol 20(22):8489–8498
Steimle V, Siegrist CA, Mottet A, Lisowska-Grospierre B, Mach B (1994) Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science 265(5168):106–109
Grzanka J, Leveson-Gower D, Golab K, Wang XJ, Marek-Trzonkowska N, Krzystyniak A et al (2013) FoxP3, Helios, and SATB1: roles and relationships in regulatory T cells. Int Immunopharmacol 16(3):343–347
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L et al (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515(7528):568–571
Chiappinelli KB, Zahnow CA, Ahuja N, Baylin SB (2016) Combining Epigenetic and Immunotherapy to Combat Cancer. Cancer Res 76(7):1683–1689
Covre A, Coral S, Di Giacomo AM, Taverna P, Azab M, Maio M (2015) Epigenetics meets immune checkpoints. Semin Oncol 42(3):506–513
Licciardi PV, Karagiannis TC (2012) Regulation of immune responses by histone deacetylase inhibitors. ISRN Hematol 2012:690901
Tellez CS, Grimes MJ, Picchi MA, Liu Y, March TH, Reed MD et al (2014) SGI-110 and entinostat therapy reduces lung tumor burden and reprograms the epigenome. Int J Cancer 135(9):2223–2231
West AC, Christiansen AJ, Smyth MJ, Johnstone RW (2012) The combination of histone deacetylase inhibitors with immune-stimulating antibodies has potent anti-cancer effects. Oncoimmunology 1(3):377–379
Fournel M, Bonfils C, Hou Y, Yan PT, Trachy-Bourget MC, Kalita A et al (2008) MGCD0103, a novel isotype-selective histone deacetylase inhibitor, has broad spectrum antitumor activity in vitro and in vivo. Mol Cancer Ther 7(4):759–768
Kepp O, Tesniere A, Schlemmer F, Michaud M, Senovilla L, Zitvogel L et al (2009) Immunogenic cell death modalities and their impact on cancer treatment. Apoptosis 14(4):364–375
Akimova T, Ge G, Golovina T, Mikheeva T, Wang L, Riley JL et al (2010) Histone/protein deacetylase inhibitors increase suppressive functions of human FOXP3+ Tregs. Clin Immunol 136(3):348–363
Li B, Samanta A, Song X, Iacono KT, Bembas K, Tao R et al (2007) FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc Natl Acad Sci USA 104(11):4571–4576
Shen L, Pili R (2012) Class I histone deacetylase inhibition is a novel mechanism to target regulatory T cells in immunotherapy. Oncoimmunology 1(6):948–950
Acknowledgements
We thank Molecular Imaging (Ann Arbor, MI) for conducting the in vivo and flow cytometry studies. We thank Active Motif (Carlsbad, CA) for assistance designing and for running ChIP-Seq studies. We thank Diane Potvin, Head of Biostatistics and Data Management and Consultant to Mirati Therapeutics for statistical analyses. We thank Dana Buckman, Flow Paradigm (San Diego, CA), for flow cytometry support. We thank Adaptive Biotechnologies (Seattle, WA) for tumor TCR sequencing and bioinformatics analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
David Briere, Niranjan Sudhakar, Jill Hallin, Lars D. Engstrom, Ruth Aranda, Harrah Chiang, Peter Olson, James G. Christensen are employees and stockholders of Mirati Therapeutics. Jeffrey S. Weber, David M. Woods and Andressa L. Sodré received research funding from Mirati Therapeutics.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Briere, D., Sudhakar, N., Woods, D.M. et al. The class I/IV HDAC inhibitor mocetinostat increases tumor antigen presentation, decreases immune suppressive cell types and augments checkpoint inhibitor therapy. Cancer Immunol Immunother 67, 381–392 (2018). https://doi.org/10.1007/s00262-017-2091-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-017-2091-y