Abstract
Purpose
The purpose of this study is to evaluate the correlation between KRAS mutation, 18F-FDG uptake, and metastatic pattern in advanced stage colorectal cancer (CRC) patients.
Methods
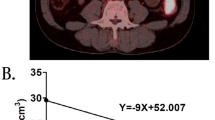
Medical records of stage IV CRC patients who underwent 18F-FDG PET/CT for staging and KRAS mutation analysis were selected. On PET scans, a volume of interest (VOI) was drawn on the primary lesion. 18F-FDG indices (SUVmax, SUVmean, MTV, TLG) of the primary lesions were obtained and correlated with KRAS mutation of the primary lesion. Also, metastatic sites were recorded. Association between metastatic pattern and KRAS expression and FDG indices were analyzed.
Results
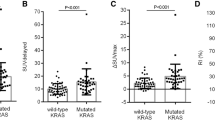
KRAS mutation was positive in 40 (43%) patients. Evaluation of FDG indices showed that higher SUVmax (14.0 vs. 11.2, p = 0.004), higher SUVmean (5.3 vs. 4.7, p = 0.005), and higher TLG (301.4 vs. 205.5, p = 0.023) were predictive of KRAS mutation compared to wild-type (WT) KRAS. Lung metastasis was more frequently involved in patients with KRAS mutation (50.0% vs. 22.6%, p = 0.006), and liver metastasis was more frequently involved in patients with WT KRAS (81.1% vs. 55.0%, p = 0.007). Multivariate analysis showed that primary tumor location (OR 3.92, p = 0.07) and KRAS mutation (OR 2.45, p = 0.09) were significant factors in lung metastasis model.
Conclusion
KRAS mutation patients had more frequent lung metastasis and had higher 18F-FDG uptake compared to WT KRAS in stage IV CRC.


Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, et al. (2015) Global cancer statistics 2012. CA Cancer J Clin 65(2):87–108
Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. (2007) Cetuximab for the treatment of colorectal cancer. N Engl J Med 357(20):2040–2048
Ku GY, Haaland BA, de Lima LopesGJr (2012) Cetuximab in the first-line treatment of K-ras wild-type metastatic colorectal cancer: the choice and schedule of fluoropyrimidine matters. Cancer Chemother Pharmacol 70(2):231–238
Lievre A, Bachet JB, Boige V, et al. (2008) KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol 26(3):374–379
Karapetis CS, Khambata-Ford S, Jonker DJ, et al. (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359(17):1757–1765
Yun J, Rago C, Cheong I, et al. (2009) Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science 325(5947):1555–1559
Kawada K, Nakamoto Y, Kawada M, et al. (2012) Relationship between 18F-fluorodeoxyglucose accumulation and KRAS/BRAF mutations in colorectal cancer. Clin Cancer Res 18(6):1696–1703
Chen SW, Chiang HC, Chen WT, et al. (2014) Correlation between PET/CT parameters and KRAS expression in colorectal cancer. Clin Nucl Med 39(8):685–689
Krikelis D, Skoura E, Kotoula V, et al. (2014) Lack of association between KRAS mutations and 18F-FDG PET/CT in Caucasian metastatic colorectal cancer patients. Anticancer Res 34(5):2571–2579
Culverwell AD, Chowdhury FU, Scarsbrook AF (2012) Optimizing the role of FDG PET-CT for potentially operable metastatic colorectal cancer. Abdom Imaging 37(6):1021–1031
Van Cutsem E, Nordlinger B, Cervantes A, et al. (2010) Advanced colorectal cancer: ESMO clinical practice guidelines for treatment. Ann Oncol 21(Suppl 5):v93–v97
Niekel MC, Bipat S, Stoker J (2010) Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology 257(3):674–684
Network NCC Clinical practice Guidelines in Oncology, Colon cancer, version 3.2015. http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf Accessed August 23, 2014
Kim MJ, Lee HS, Kim JH, et al. (2012) Different metastatic pattern according to the KRAS mutational status and site-specific discordance of KRAS status in patients with colorectal cancer. BMC Cancer 12:347
Cejas P, Lopez-Gomez M, Aguayo C, et al. (2009) KRAS mutations in primary colorectal cancer tumors and related metastases: a potential role in prediction of lung metastasis. PLoS ONE 4(12):e8199
Iwamoto M, Kawada K, Nakamoto Y, et al. (2014) Regulation of 18F-FDG accumulation in colorectal cancer cells with mutated KRAS. J Nucl Med 55(12):2038–2044
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was supported by the Grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A110853).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
The institutional review board approved this retrospective study. Informed consent was waived.
Rights and permissions
About this article
Cite this article
Cho, A., Jo, K., Hwang, S.H. et al. Correlation between KRAS mutation and 18F-FDG uptake in stage IV colorectal cancer. Abdom Radiol 42, 1621–1626 (2017). https://doi.org/10.1007/s00261-017-1054-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1054-2