Abstract
Purpose
Amyloid PET which has been widely used for noninvasive assessment of cortical amyloid burden is visually interpreted in the clinical setting. As a fast and easy-to-use visual interpretation support system, we analyze whether the deep learning–based end-to-end estimation of amyloid burden improves inter-reader agreement as well as the confidence of the visual reading.
Methods
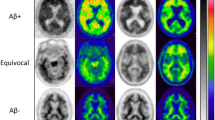
A total of 121 clinical routines [18F]Florbetaben PET images were collected for the randomized blind-reader study. The amyloid PET images were visually interpreted by three experts independently blind to other information. The readers qualitatively interpreted images without quantification at the first reading session. After more than 2-week interval, the readers additionally interpreted images with the quantification results provided by the deep learning system. The qualitative assessment was based on a 3-point BAPL score (1: no amyloid load, 2: minor amyloid load, and 3: significant amyloid load). The confidence score for each session was evaluated by a 3-point score (0: ambiguous, 1: probably, and 2: definite to decide).
Results
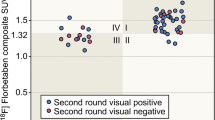
Inter-reader agreements for the visual reading based on a 3-point scale (BAPL score) calculated by Fleiss kappa coefficients were 0.46 and 0.76 for the visual reading without and with the deep learning system, respectively. For the two reading sessions, the confidence score of visual reading was improved at the visual reading session with the output (1.27 ± 0.078 for visual reading-only session vs. 1.66 ± 0.63 for a visual reading session with the deep learning system).
Conclusion
Our results highlight the impact of deep learning–based one-step amyloid burden estimation system on inter-reader agreement and confidence of reading when applied to clinical routine amyloid PET reading.



Similar content being viewed by others
Data availability
Data that support this study can be made available upon reasonable request.
References
2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020. doi:https://doi.org/10.1002/alz.12068.
Bacskai BJ, Kajdasz ST, Christie RH, Carter C, Games D, Seubert P, et al. Imaging of amyloid-beta deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat Med. 2001;7:369–72. https://doi.org/10.1038/85525.
Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–31. https://doi.org/10.1002/ana.21843.
Jack CR Jr, Barrio JR, Kepe V. Cerebral amyloid PET imaging in Alzheimer’s disease. Acta Neuropathol. 2013;126:643–57. https://doi.org/10.1007/s00401-013-1185-7.
Dementia key facts. WHO. 21. MAR ed; 2019.
Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. Jama-J Am Med Assoc. 2011;305:275–83. https://doi.org/10.1001/jama.2010.2008.
Minoshima S, Drzezga AE, Barthel H, Bohnen N, Djekidel M, Lewis DH, et al. SNMMI procedure standard/EANM practice guideline for amyloid PET imaging of the brain 1.0. J Nucl Med. 2016;57:1316–22. https://doi.org/10.2967/jnumed.116.174615.
Nayate AP, Dubroff JG, Schmitt JE, Nasrallah I, Kishore R, Mankoff D, et al. Use of standardized uptake value ratios decreases interreader variability of [F-18]florbetapir PET brain scan interpretation. Am J Neuroradiol. 2015;36:1237–44. https://doi.org/10.3174/ajnr.A4281.
Pontecorvo MJ, Arora AK, Devine M, Lu M, Galante N, Siderowf A, et al. Quantitation of PET signal as an adjunct to visual interpretation of florbetapir imaging. Eur J Nucl Med Mol I. 2017;44:825–37. https://doi.org/10.1007/s00259-016-3601-4.
Alongi P, Sardina DS, Coppola R, Scalisi S, Puglisi V, Arnone A, et al. 18F-Florbetaben PET/CT to assess Alzheimer’s disease: a new analysis method for regional amyloid quantification. J Neuroimaging. 2019;29:383–93. https://doi.org/10.1111/jon.12601.
Bourgeat P, Villemagne VL, Dore V, Brown B, Macaulay SL, Martins R, et al. Comparison of MR-less PiB SUVR quantification methods. Neurobiol Aging. 2015;36(Suppl 1):S159–66. https://doi.org/10.1016/j.neurobiolaging.2014.04.033.
Bourgeat P, Dore V, Fripp J, Villemagne V, Rowe C, Salvado O. Computational analysis of PET by AIBL (CapAIBL): a cloud-based processing pipeline for the quantification of PET images. J Nucl Med. 2015;56.
Zhou L, Salvado O, Dore V, Bourgeat P, Raniga P, Macaulay SL, et al. MR-less surface-based amyloid assessment based on 11C PiB PET. PLoS One. 2014;9:e84777. https://doi.org/10.1371/journal.pone.0084777.
Kim JY, Suh HY, Ryoo HG, Oh D, Choi H, Paeng JC, et al. Amyloid PET quantification via end-to-end training of a deep learning. Nucl Med Molec Imag. 2019;53:340–8. https://doi.org/10.1007/s13139-019-00610-0.
Kim JP, Kim J, Kim Y, Moon SH, Park YH, Yoo S, et al. Staging and quantification of florbetaben PET images using machine learning: impact of predicted regional cortical tracer uptake and amyloid stage on clinical outcomes. Eur J Nucl Med Mol Imaging. 2020;47:1971–83. https://doi.org/10.1007/s00259-019-04663-3.
American Psychiatric Association., American Psychiatric Association. Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders : DSM-IV. 4th ed. Washington, DC: American Psychiatric Association; 1994.
Mckhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical-diagnosis of Alzheimers-disease - report of the Nincds-Adrda Work Group under the Auspices of Department-of-Health-and-Human-Services Task-Force on Alzheimers-Disease. Neurology. 1984;34:939–44. https://doi.org/10.1212/Wnl.34.7.939.
Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia - diagnostic-criteria for research studies - report of the Ninds-Airen International Workshop. Neurology. 1993(43):250–60. https://doi.org/10.1212/Wnl.43.2.250.
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies - third report of the DLB consortium. Neurology. 2005;65:1863–72. https://doi.org/10.1212/01.wnl.0000187889.17253.b1.
Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. https://doi.org/10.1212/wnl.51.6.1546.
Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–6. https://doi.org/10.1111/j.1365-2796.2004.01380.x.
Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FRJ, et al. Prevalence of cerebral amyloid pathology in persons without dementia a meta-analysis. Jama-J Am Med Assoc. 2015;313:1924–38. https://doi.org/10.1001/jama.2015.4668.
Sabri O, Seibyl J, Rowe C, Barthel H. Beta-amyloid imaging with florbetaben. Clin Transl Imaging. 2015;3:13–26. https://doi.org/10.1007/s40336-015-0102-6.
Landau SM, Fero A, Baker SL, Koeppe R, Mintun M, Chen K, et al. Measurement of longitudinal beta-amyloid change with 18F-florbetapir PET and standardized uptake value ratios. J Nucl Med. 2015;56:567–74. https://doi.org/10.2967/jnumed.114.148981.
Payoux P, Delrieu J, Gallini A, Adel D, Salabert AS, Hitzel A, et al. Cognitive and functional patterns of nondemented subjects with equivocal visual amyloid PET findings. Eur J Nucl Med Mol Imaging. 2015;42:1459–68. https://doi.org/10.1007/s00259-015-3067-9.
Barthel H, Sabri O. Clinical use and utility of amyloid imaging. J Nucl Med. 2017;58:1711–7. https://doi.org/10.2967/jnumed.116.185017.
Oh M, Seo M, Oh SY, Kim H, Choi BW, Oh JS, et al. Clinical significance of visually equivocal amyloid PET findings from the Alzheimer’s Disease Neuroimaging Initiative cohort. Neuroreport. 2018;29:553–8. https://doi.org/10.1097/Wnr.0000000000000986.
Okada Y, Kato T, Iwata K, Kimura Y, Nakamura A, Hattori H, et al. Evaluation of PiB visual interpretation with CSF A beta and longitudinal SUVR in J-ADNI study. Ann Nucl Med. 2020;34:108–18. https://doi.org/10.1007/s12149-019-01420-2.
Yamane T, Ishii K, Sakata M, Ikari Y, Nishio T, Ishii K, et al. Inter-rater variability of visual interpretation and comparison with quantitative evaluation of (11)C-PiB PET amyloid images of the Japanese Alzheimer’s Disease Neuroimaging Initiative (J-ADNI) multicenter study. Eur J Nucl Med Mol Imaging. 2017;44:850–7. https://doi.org/10.1007/s00259-016-3591-2.
Harn NR, Hunt SL, Hill J, Vidoni E, Perry M, Burns JM. Augmenting amyloid PET interpretations with quantitative information improves consistency of early amyloid detection. Clin Nucl Med. 2017;42:577–81. https://doi.org/10.1097/RLU.0000000000001693.
Cho SH, Choe YS, Kim HJ, Jang H, Kim Y, Kim SE, et al. A new Centiloid method for (18)F-florbetaben and (18)F-flutemetamol PET without conversion to PiB. Eur J Nucl Med Mol Imaging. 2020;47:1938–48. https://doi.org/10.1007/s00259-019-04596-x.
Klunk WE, Koeppe RA, Price JC, Benzinger TL, Devous MD Sr, Jagust WJ, et al. The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 2015;11:1–15 e1–4. https://doi.org/10.1016/j.jalz.2014.07.003.
Becker JA, Hedden T, Carmasin J, Maye J, Rentz DM, Putcha D, et al. Amyloid-beta associated cortical thinning in clinically normal elderly. Ann Neurol. 2011;69:1032–42. https://doi.org/10.1002/ana.22333.
Ripolles P, Marco-Pallares J, de Diego-Balaguer R, Miro J, Falip M, Juncadella M, et al. Analysis of automated methods for spatial normalization of lesioned brains. Neuroimage. 2012;60:1296–306. https://doi.org/10.1016/j.neuroimage.2012.01.094.
Reig S, Penedo M, Gispert JD, Pascau J, Sanchez-Gonzalez J, Garcia-Barreno P, et al. Impact of ventricular enlargement on the measurement of metabolic activity in spatially normalized PET. Neuroimage. 2007;35:748–58. https://doi.org/10.1016/j.neuroimage.2006.12.015.
van Westen D, Lindqvist D, Blennow K, Minthon L, Nagga K, Stomrud E, et al. Cerebral white matter lesions - associations with Abeta isoforms and amyloid PET. Sci Rep. 2016;6:20709. https://doi.org/10.1038/srep20709.
Johnson VE, Stewart W, Smith DH. Traumatic brain injury and amyloid-beta pathology: a link to Alzheimer’s disease? Nat Rev Neurosci. 2010;11:361–70. https://doi.org/10.1038/nrn2808.
Rabinovici GD, Gatsonis C, Apgar C, Chaudhary K, Gareen I, Hanna L, et al. Association of amyloid positron emission tomography with subsequent change in clinical management among Medicare beneficiaries with mild cognitive impairment or dementia. Jama-J Am Med Assoc. 2019;321:1286–94. https://doi.org/10.1001/jama.2019.2000.
Funding
This research was financially supported by the National Research Foundation of Korea Grant funded by the Korea Government (No. NRF-2019K1A3A1A14065446 and NRF-2019R1F1A1061412).
Author information
Authors and Affiliations
Contributions
H.C. designed the study. J.Y.K. performed image analysis. D.Y.L and K.S. contributed to collect and analyze clinical data. J.Y.K., D.O., and H.C. contributed to the image interpretation. J.Y.K. and D.O. contributed to the data collection and literature review. J.C.P., G.J.C., K.W.K., and D.S.L. contributed to data interpretation and analysis. J.Y.K. and H.C. wrote the manuscript mainly and all authors critically reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were following the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent to clinical testing and neuroimaging approved by the institutional review boards of participating institutions (SNUH IRB Registration Number 2004-047-1116).
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology
Rights and permissions
About this article
Cite this article
Kim, JY., Oh, D., Sung, K. et al. Visual interpretation of [18F]Florbetaben PET supported by deep learning–based estimation of amyloid burden. Eur J Nucl Med Mol Imaging 48, 1116–1123 (2021). https://doi.org/10.1007/s00259-020-05044-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-05044-x