Abstract
Purpose
Tau accumulation is a core pathologic change in various neurodegenerative diseases including Alzheimer’s disease and frontotemporal lobar degeneration-tau. Recently, tau positron emission tomography tracers such as [18F] AV-1451 and [18F] THK5351 have been developed to detect tau deposition in vivo. In the present study, we performed a head to head comparison of these two tracers in Alzheimer’s disease and frontotemporal dementia cases and aimed to investigate which tracers are better suited to image tau in these disorders.
Methods
A cross-sectional study was conducted using a hospital-based sample at a tertiary referral center. We recruited eight participants (two Alzheimer’s disease, four frontotemporal dementia and two normal controls) who underwent magnetic resonance image, amyloid positron emission tomography with [18F]-Florbetaben and tau positron emission tomography with both THK5351 and AV-1451. To measure regional AV1451 and THK5351 uptakes, we used the standardized uptake value ratios by dividing mean activity in target volume of interest by mean activity in the cerebellar hemispheric gray matter.
Results
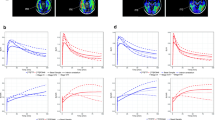
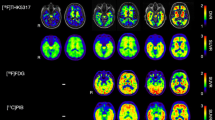
Although THK5351 and AV-1451 uptakes were highly correlated, cortical uptake of AV-1451 was more striking in Alzheimer’s disease, while cortical uptake of THK5351 was more prominent in frontotemporal dementia. THK5351 showed higher off-target binding than AV-1451 in the white matter, midbrain, thalamus, and basal ganglia.
Conclusions
AV-1451 is more sensitive and specific to Alzheimer’s disease type tau and shows lower off-target binding, while THK5351 may mirror non-specific neurodegeneration.



Similar content being viewed by others
References
Trojanowski JQ, Clark CM, Schmidt ML, Arnold SE, Lee VM. Strategies for improving the postmortem neuropathological diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997;18:S75–9.
Mackenzie IR, Foti D, Woulfe J, Hurwitz TA. Atypical frontotemporal lobar degeneration with ubiquitin-positive, TDP-43-negative neuronal inclusions. Brain. 2008;131:1282–93.
Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain. 2005;128:1996–2005.
Rascovsky K, Hodges J, Knopman D, Mendez M, Kramer J, Neuhaus J, et al. Can clinical features predict tau pathology in patients with behavioral variant frontotemporal dementia? Annual meeting of the American-Academy-of-Neurology. 2013.
Ariza M, Kolb HC, Moechars D, Rombouts F, Andres JI. Tau positron emission tomography (PET) imaging: past, present, and future. J Med Chem. 2015;58:4365–82.
Dickson DW, Kouri N, Murray ME, Josephs KA. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J Mol Neurosci. 2011;45:384–9.
Okamura N, Furumoto S, Harada R, Tago T, Yoshikawa T, Fodero-Tavoletti M, et al. Novel 18F-labeled arylquinoline derivatives for noninvasive imaging of tau pathology in Alzheimer disease. J Nucl Med. 2013;54:1420–7.
Okamura N, Furumoto S, Furukawa K, Ishiki A, Harada R, Iwata R, et al. PET imaging of tau pathology in mild cognitive impairment and Alzheimer’s disease with [18F]THK-5351. Annual meeting of the Society-of-Nuclear-Medicine-and-Molecular-Imaging. 2015.
Ishiki A, Harada R, Okamura N, Tomita N, Rowe CC, Villemagne VL, et al. Tau imaging with [18 F]THK-5351 in progressive supranuclear palsy. Eur J Neurol. 2016.
Xia CF, Arteaga J, Chen G, Gangadharmath U, Gomez LF, Kasi D, et al. [(18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimers Dement. 2013;9:666–76.
Cho H, Choi JY, Hwang MS, Kim YJ, Lee HM, Lee HS, et al. In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann Neurol. 2016;80:247–58.
Chien DT, Bahri S, Szardenings AK, Walsh JC, Mu F, Su MY, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimers Dis. 2013;34:457–68.
Cho H, Choi JY, Hwang MS, Lee JH, Kim YJ, Lee HM, et al. Tau PET in Alzheimer disease and mild cognitive impairment. Neurology. 2016;87:375–83.
Lockhart SN, Baker SL, Okamura N, Furukawa K, Ishiki A, Furumoto S, et al. Dynamic PET measures of tau accumulation in cognitively normal older adults and Alzheimer’s disease patients measured using [18F] THK-5351. PLoS One. 2016;11:e0158460.
Hansen AK, Knudsen K, Lillethorup TP, Landau AM, Parbo P, Fedorova T, et al. In vivo imaging of neuromelanin in Parkinson’s disease using 18F-AV-1451 PET. Brain. 2016;139:2039–49.
Marquie M, Normandin MD, Meltzer AC, Siao Tick Chong M, Andrea NV, Anton-Fernandez A, et al. Pathological correlations of [F-18]-AV-1451 imaging in non-alzheimer tauopathies. Ann Neurol. 2017;81:117–28.
Marquie M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 2015;78:787–800.
Lee MK, Hwang BY, Lee SA, Oh GJ, Choi WH, Hong SS, et al. 1-methyl-2-undecyl-4(1H)-quinolone as an irreversible and selective inhibitor of type B monoamine oxidase. Chem Pharm Bull (Tokyo). 2003;51:409–11.
Ng KP, Massarweh G, Soucy JP, Gravel P, Pascoal TA, Mathotaarachchi S, et al. Selegiline reduces brain [18F]THK5351 binding. 11th Human Amyloid Imaging. 2017;187.
Rowe CC, Ackerman U, Browne W, Mulligan R, Pike KL, O’Keefe G, et al. Imaging of amyloid beta in Alzheimer’s disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol. 2008;7:129–35.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9.
Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–77.
Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14.
Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54.
Barthel H, Luthardt J, Becker G, Patt M, Hammerstein E, Hartwig K, et al. Individualized quantification of brain beta-amyloid burden: results of a proof of mechanism phase 0 florbetaben PET trial in patients with Alzheimer’s disease and healthy controls. Eur J Nucl Med Mol Imaging. 2011;38:1702–14.
Collins DL, Holmes CJ, Peters TM, Evans AC. Automatic 3-D model-based neuroanatomical segmentation. Hum Brain Mapp. 1995;3:190–208.
Collins DL, Zijdenbos AP, Baaré WFC, Evans AC. ANIMAL+INSECT: Improved cortical structure segmentation. In: Kuba A, Šáamal M, Todd-Pokropek A, editors. Information processing in medical imaging: 16th international conference, IPMI’99 Visegrád, Hungary, June 28 – July 2, 1999 Proceedings. Berlin, Heidelberg: Springer Berlin Heidelberg; 1999. p. 210–23.
Martersteck A, Sridhar J, Rainford A, Mesulam M, Rogalski E. Recovering signal from AV1451 in frontotemporal lobar degeneration with partial volume correction. 11th Human Amyloid Imaging; 2017. p. 176.
Sander K, Lashley T, Gami P, Gendron T, Lythgoe MF, Rohrer JD, et al. Characterization of tau positron emission tomography tracer [18F]AV-1451 binding to postmortem tissue in Alzheimer’s disease, primary tauopathies, and other dementias. Alzheimers Dement. 2016;12:1116–24.
Lowe VJ, Curran G, Fang P, Liesinger AM, Josephs KA, Parisi JE, et al. An autoradiographic evaluation of AV-1451 tau PET in dementia. Acta Neuropathol Commun. 2016;4:58.
Smith R, Puschmann A, Scholl M, Ohlsson T, van Swieten J, Honer M, et al. 18F-AV-1451 tau PET imaging correlates strongly with tau neuropathology in MAPT mutation carriers. Brain. 2016;139:2372–9.
Spina S, Schonhaut DR, Boeve BF, Seeley WW, Ossenkoppele R, O’Neil JP, et al. Frontotemporal dementia with the V337M MAPT mutation: tau-PET and pathology correlations. Neurology. 2017.
Hwang J, Kim JE, Park S-H, Roh JH, Lee J-H. A Non-fluent agrammatic primary progressive aphasia documented with an 18F–THK5351-PET. 2016 Annual Spring Meeting of Korean Dementia Association; 2016.
Lee H, Lee S-Y, Jeong HJ, Woo S-H, Lee K-M, Lee Y-B, et al. Tau PET imaging in patients with semantic variant primary progressive aphasia 35th 2016 Annual Autumn Meeting of the Korean Neurological Association; 2016.
Kim JE, Hwang J, Park S-H, Oh M, Kim JS, Lee J-H, et al. 18F–THK5351 PET findings indicatige of brain injury rather than presumtive pathology in FTLD. 35th 2016 Annual Autumn Meeting of the Korean Neurological Association; 2016. p. 171.
Hodges JR, Mitchell J, Dawson K, Spillantini MG, Xuereb JH, McMonagle P, et al. Semantic dementia: demography, familial factors and survival in a consecutive series of 100 cases. Brain. 2010;133:300–6.
Snowden J, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol. 2007;114:31–8.
Kim JE, Hwang J, Park S-H, Ko MA, Lee Y, Lee J-H, et al. Combined [18F]Florbetaben Amyloid PET and [18F]THK-5351 Tau PET as a potential pathfinder in a patient with rapidly progressive dementia. 2016 Annual Spring Meeting of the Korean Dementia Association; 2016. p. 165.
Kawamoto Y, Akiguchi I, Jarius C, Budka H. Enhanced expression of 14-3-3 proteins in reactive astrocytes in Creutzfeldt-Jakob disease brains. Acta Neuropathol. 2004;108:302–8.
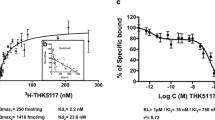
Ng KP, Pascoal TA, Mathotaarachchi S, Therriault J, Kang MS, Shin M, et al. Monoamine oxidase B inhibitor, selegiline, reduces 18F-THK5351 uptake in the human brain. Alzheimers Res Ther. 2017;9:25.
Bevan-Jones WR, Cope TE, Jones PS, Passamonti L, Hong YT, Fryer TD, et al. [18F]AV-1451 binding in vivo mirrors the expected distribution of TDP-43 pathology in the semantic variant of primary progressive aphasia. J Neurol Neurosurg Psychiatry. 2017.
Hostetler ED, Walji AM, Zeng Z, Miller P, Bennacef I, Salinas C, et al. Preclinical characterization of 18F-MK-6240, a promising PET tracer for in vivo quantification of human Neurofibrillary tangles. J Nucl Med. 2016;57:1599–606.
Fowler JS, Volkow ND, Wang GJ, Logan J, Pappas N, Shea C, et al. Age-related increases in brain monoamine oxidase B in living healthy human subjects. Neurobiol Aging. 1997;18:431–5.
Aquino D, Bizzi A, Grisoli M, Garavaglia B, Bruzzone MG, Nardocci N, et al. Age-related iron deposition in the basal ganglia: quantitative analysis in healthy subjects. Radiology. 2009;252:165–72.
Massey LA, Miranda MA, Al-Helli O, Parkes HG, Thornton JS, So PW, et al. 9.4 T MR microscopy of the substantia nigra with pathological validation in controls and disease. Neuroimage Clin. 2017;13:154–63.
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI14C2768); and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. NRF-2017R1A2B2005081).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
G.D.R. receives research support from Avid Radiopharmaceuticals, Eli Lilly, GE Healthcare and Piramal. He has received consulting fees and speaking honoraria from Eisai, Genentech, Roche, Lundbeck, Putnam and Merck.
Role of the funder
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Jang, Y.K., Lyoo, C.H., Park, S. et al. Head to head comparison of [18F] AV-1451 and [18F] THK5351 for tau imaging in Alzheimer’s disease and frontotemporal dementia. Eur J Nucl Med Mol Imaging 45, 432–442 (2018). https://doi.org/10.1007/s00259-017-3876-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3876-0