Abstract
Introduction
Mastocytosis is a clonal haematological disease characterized by uncontrolled proliferation and the activation of mast cells. The value of FDG-PET/CT (FDG-PET) in mastocytosis has yet to be determined.
Methods
We retrospectively identified patients with an established diagnosis of systemic mastocytosis (SM), according to the WHO criteria, who underwent PET using the French Reference Centre for Mastocytosis database. Semi-quantitative and visual analysis of FDG-PET was performed and compared to the clinico-biological data.
Results
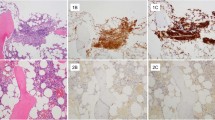
Our cohort included 19 adult patients, median age 65 years [range 58–74], including three with smouldering SM (SSM), three with aggressive SM (ASM), 10 with an associated clonal haematological non-mast-cell lineage disease (SM-AHNMD), and three with mast cell sarcoma (MCS). FDG-PET was performed at the time of the SM diagnosis (15/19), to evaluate lymph node (LN) activity (3/19) or the efficacy of therapy (1/19). FDG uptake was observed in the bone marrow (BM) (9/19, 47 %), LN (6/19, 32 %), spleen (12/19, 63 %), or liver (1/19, 5 %). No significant FDG uptake was observed in the SSM and ASM patients. A pathological FDG uptake was observed in the BM of 6/10 patients with SM-AHNMD, appearing as diffuse and homogeneous, and in the LN of 5/10 patients. All 3 MCS patients showed intense and multifocal BM pathological uptake, mimicking metastasis. No correlation was found between the FDG-PET findings and serum tryptase levels, BM mast cell infiltration percentage, and CD30 and CD2 expression by mast cells.
Conclusions
FDG uptake does not appear to be a sensitive marker of mast cell activation or proliferation because no significant FDG uptake was observed in most common forms of mastocytosis (notably purely aggressive SM). However, pathological FDG uptake was observed in the SM-AHNMD and in MCS cases, suggesting a role of FDG-PET in their early identification and as a tool of therapeutic assessment in this subgroup of patients.


Similar content being viewed by others
References
Fain O, Stirnemann J, Lortholary O. Systemic mastocytosis. Rev Prat. 2005;55(15):1629–36.
Gotlib J, Pardanani A, Akin C, Reiter A, George T, Hermine O, et al. International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) & European Competence Network on Mastocytosis (ECNM) consensus response criteria in advanced systemic mastocytosis. Blood. 2013;121(13):2393–401.
Valent P, Akin C, Arock M, Brockow K, Butterfield JH, Carter MC, et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 2012;157(3):215–25.
Valent P, Horny HP, Escribano L, Longley BJ, Li CY, Schwartz LB, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25(7):603–25.
Biggi A, Gallamini A, Chauvie S, Hutchings M, Kostakoglu L, Gregianin M, et al. International validation study for interim PET in ABVD-treated, advanced-stage Hodgkin Lymphoma: Interpretation Criteria and Concordance Rate Among Reviewers. J Nucl Med. 2013.
Hutchings M. How does PET/CT help in selecting therapy for patients with Hodgkin lymphoma? Hematol Am Soc Hematol Educ Program. 2012;2012:322–7.
Moskowitz CH. Interim PET-CT in the management of diffuse large B-cell lymphoma. Hematol Am Soc Hematol Educ Program. 2012;2012:397–401.
Inoue K, Okada K, Harigae H, Taki Y, Goto R, Kinomura S, et al. Diffuse bone marrow uptake on F-18 FDG PET in patients with myelodysplastic syndromes. Clin Nucl Med. 2006;31(11):721–3.
Nakajo M, Jinnouchi S, Inoue H, Otsuka M, Matsumoto T, Kukita T, et al. FDG PET findings of chronic myeloid leukemia in the chronic phase before and after treatment. Clin Nucl Med. 2007;32(10):775–8.
Burrell SC, Fischman AJ. Myelofibrosis on F-18 FDG PET imaging. Clin Nucl Med. 2005;30(10):674.
Zettinig G, Becherer A, Szabo M, Uffmann M, Dudczak R, Valent P, et al. FDG positron emission tomography in patients with systemic mastocytosis. AJR Am J Roentgenol. 2002;179(5):1235–7.
Kim Y, Weiss LM, Chen YY, Pullarkat V. Distinct clonal origins of systemic mastocytosis and associated B-cell lymphoma. Leuk Res. 2007;31(12):1749–54.
Tomihama RT, McEachen JC, Kuo PH. Imaging of systemic mastocytosis by FDG-PET/CT demonstrates increased activity in cortical bone. Clin Nucl Med. 2008;33(3):220–3.
Valent P, Sotlar K, Horny HP. Aberrant expression of CD30 in aggressive systemic mastocytosis and mast cell leukemia: a differential diagnosis to consider in aggressive hematopoietic CD30-positive neoplasms. Leuk Lymphoma. 2011;52(5):740–4.
Dong A, Wang Y, Gao L, Zuo C. 18F-FDG PET/CT findings in a patient with sweet syndrome associated with myelodysplastic syndrome. Clin Nucl Med. 2013.
Brown RS, Leung JY, Fisher SJ, Frey KA, Ethier SP, Wahl RL. Intratumoral distribution of tritiated fluorodeoxyglucose in breast carcinoma: I. Are inflammatory cells important? J Nucl Med. 1995;36(10):1854–61.
Fritz J, Fishman EK, Carrino JA, Horger MS. Advanced imaging of skeletal manifestations of systemic mastocytosis. Skeletal Radiol. 2012;41(8):887–97.
Agool A, Glaudemans AW, Boersma HH, Dierckx RA, Vellenga E, Slart RH. Radionuclide imaging of bone marrow disorders. Eur J Nucl Med Mol Imaging. 2010;38(1):166–78.
Vanderhoek M, Juckett MB, Perlman SB, Nickles RJ, Jeraj R. Early assessment of treatment response in patients with AML using [(18)F]FLT PET imaging. Leuk Res. 2010;35(3):310–6.
Dankerl A, Liebisch P, Glatting G, Friesen C, Blumstein NM, Kocot D, et al. Multiple myeloma: molecular imaging with 11C-methionine PET/CT–initial experience. Radiology. 2007;242(2):498–508.
Itti E, Meignan M, Berriolo-Riedinger A, Biggi A, Cashen AF, Vera P, et al. An international confirmatory study of the prognostic value of early PET/CT in diffuse large B-cell lymphoma: comparison between Deauville criteria and DeltaSUVmax. Eur J Nucl Med Mol Imaging. 2013;40(9):1312–20.
Acknowledgments
We would like to thank Isabelle Hirsch and Laure Cabaret from CEREMAST for their work as clinical trial associates.
Compliance with ethical standards
ᅟ
Conflict of interest
The authors declare that they have no conflict of interest.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent was obtained from all individual participants included in the study
Sources of support
No financial support was received.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Djelbani-Ahmed and M. O. Chandesris contributed equally to this work.
Rights and permissions
About this article
Cite this article
Djelbani-Ahmed, S., Chandesris, M.O., Mekinian, A. et al. FDG-PET/CT findings in systemic mastocytosis: a French multicentre study. Eur J Nucl Med Mol Imaging 42, 2013–2020 (2015). https://doi.org/10.1007/s00259-015-3117-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3117-3