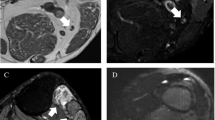
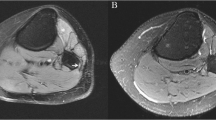
Abstract
Objective
To evaluate the effect of intravenous (IV) contrast on sensitivity, specificity, and accuracy of magnetic resonance (MR) neurography of the knee with attention to the common peroneal nerve (CPN) in identifying nerve lesions and active muscle denervation changes.
Materials and methods
A retrospective search for contrast-enhanced MR neurography cases evaluating the CPN at the knee was performed. Patients with electrodiagnostic testing (EDX) within 3 months of imaging were included and those with relevant prior surgery were excluded. Two radiologists independently reviewed non-contrast sequences and then 4 weeks later evaluated non-contrast and contrast sequences. McNemar’s tests were performed to detect a difference between non-contrast only and combined non-contrast and contrast sequences in identifying nerve lesions and active muscle denervation changes using EDX as the reference standard.
Results
Forty-four exams in 42 patients (2 bilateral) were included. Twenty-eight cases had common peroneal neuropathy and 29, 21, and 9 cases had active denervation changes in the anterior, lateral, and posterior compartment/proximal muscles respectively on EDX. Sensitivity, specificity, and accuracy of non-contrast versus combined non-contrast and contrast sequences for common peroneal neuropathy were 50.0%, 56.2%, and 52.3% versus 50.0%, 56.2%, and 52.3% for reader 1 and 57.1%, 50.0%, and 54.5% versus 64.3%, 56.2%, and 61.4% for reader 2. Sensitivity, specificity, and accuracy of non-contrast and combined non-contrast and contrast sequences in identifying active denervation changes for anterior, lateral, and posterior compartment muscles were not significantly different. McNemar’s tests were all negative.
Conclusion
IV contrast does not improve the ability of MR neurography to detect CPN lesions or active muscle denervation changes.





Similar content being viewed by others
References
Newhart H, Patterson J, Gunasekaran A, Pandey T, Kumar M, Kazemi N. The incremental value of magnetic resonance neurography for the neurosurgeon: review of the literature. World Neurosurg. 2019;122:331–41.
Chhabra A, Belzberg AJ, Rosson GD, et al. Impact of high resolution 3 tesla MR neurography (MRN) on diagnostic thinking and therapeutic patient management. Eur Radiol. 2016;26(5):1235–44.
Chhabra A, Andreisek G, Soldatos T, et al. MR neurography: past, present, and future. Am J Roentgenol. 2011;197(3):583–91.
Sneag DB, Saltzman EB, Meister DW, Feinberg JH, Lee SK, Wolfe SW. MRI bullseye sign: an indicator of peripheral nerve constriction in Parsonage-Turner syndrome. Muscle Nerve. 2017;56(1):99–106.
Sneag DB, Arányi Z, Zusstone EM, et al. Fascicular constrictions above elbow typify anterior interosseous nerve syndrome. Muscle Nerve. 2020;61(3):301–10.
Sneag DB, Queler S. Technological advancements in magnetic resonance neurography. Curr Neurol Neurosci Rep. 2019;19(10):75.
Deshmukh S, Carrino JA, Feinberg JH, Wolfe SW, Eagle S, Sneag DB. Pins and needles from fingers to toes: high-resolution MRI of peripheral sensory mononeuropathies. Am J Roentgenol. 2017;208(1):W1-10.
Sneag DB, Rancy SK, Wolfe SW, Lee SC, Kalia V, Lee SK, et al. Brachial plexitis or neuritis? MRI features of lesion distribution in Parsonage-Turner syndrome. Muscle Nerve. 2018;58(3):359–66.
Chhabra A, Subhawong TK, Bizzell C, Flammang A, Soldatos T. 3T MR neurography using three-dimensional diffusion-weighted PSIF: technical issues and advantages. Skeletal Radiol. 2011;40(10):1355–60.
Noguerol TM, Barousse R, Cabrera MG, Socolovsky M, Bencardino JT, Luna A. Functional MR neurography in evaluation of peripheral nerve trauma and postsurgical assessment. Radiographics. 2019;39(2):427–46.
Chhabra A, Bajaj G, Wadhwa V, et al. MR neurographic evaluation of facial and neck pain: normal and abnormal craniospinal nerves below the skull base. Radiographics. 2018;38(5):1498–513.
Daniels SP, Feinberg JH, Carrino JA, Behzadi AH, Sneag DB. MRI of foot drop: how we do it. Radiology. 2018;289(1):9–24.
Chhabra A, Chalian M, Soldatos T, et al. 3-T high-resolution MR neurography of sciatic neuropathy. Am J Roentgenol. 2012;198(4):357–64.
Jeon T, Fung MM, Koch KM, Tan ET, Sneag DB. Peripheral nerve diffusion tensor imaging: overview, pitfalls, and future directions. J Magn Reson Imaging. 2018;47(5):1171–89.
Chalian M, Chhabra A. Top-10 tips for getting started with magnetic resonance neurography. Semin Musculoskelet Radiol. 2019;23(4):347–60.
Harrell AD, Johnson D, Samet J, Omar IM, Deshmukh S. With or without? A retrospective analysis of intravenous contrast utility in magnetic resonance neurography. Skeletal Radiol. 2019;49(4):577–84.
Thawait SK, Chaudhry V, Thawait GK, et al. High-resolution mr neurography of diffuse peripheral nerve lesions. Am J Neuroradiol. 2011;32(8):1365–72.
Andreisek G, Burg D, Studer A, Weishaupt D. Upper extremity peripheral neuropathies: role and impact of mr imaging on patient management. Eur Radiol. 2008;18(9):1953–61.
Rubin GD. Costing in radiology and health care: rationale, relativity, rudiments, and realities. Radiology. 2017;282(2):333–47.
Levine D, McDonald RJ, Kressel HY. Gadolinium retention after contrast-enhanced MRI. JAMA. 2018;320(18):1853–4.
Jacobs PM, Weinreb J, Ellis JH, et al. Gadolinium retention: a research roadmap from the 2018 NIH/ACR/RSNA workshop on gadolinium chelates. Radiology. 2018;289(2):517–34.
Sailer AM, van Zwam WH, Wildberger JE, Grutters JPC. Cost-effectiveness modelling in diagnostic imaging: a stepwise approach. Eur Radiol. 2015;25(12):3629–37.
Gielen JLMA, De Schepper AM, Vanhoenacker F, et al. Accuracy of MRI in characterization of soft tissue tumors and tumor-like lesions. a prospective study in 548 patients. Eur Radiol. 2004;14(12):2320–30.
Beltran J, Chandnani V, McGhee RA, Kursunoglu-Brahme S. Gadopentetate dimeglumine-enhanced MR imaging of the musculoskeletal system. Am J Roentgenol. 1991;156(3):457–66.
Lee PP, Chalian M, Bizzell C, et al. Magnetic resonance neurography of common peroneal (fibular) neuropathy. J Comput Assist Tomogr. 2012;36(4):455–61.
Del GF, Santini F, Herzka DA, et al. Fat-suppression techniques for 3-T MR imaging of the musculoskeletal system. Radiographics. 2014;34(1):217–33.
Chhabra A, Faridian-Aragh N, Chalian M, et al. High-resolution 3-T mr neurography of peroneal neuropathy. Skeletal Radiol. 2012;41(3):257–71.
Wang X, Harrison C, Mariappan YK, Madhuranthakam AJ. MR neurography of brachial plexus at 3.0 T with robust fat and blood suppression. Radiology. 2016;283(2):538–46.
Kästel T, Heiland S, Bäumer P, Bartsch AJ, Bendszus M, Pham M. Magic angle effect: a relevant artifact in MR neurography at 3T? Am J Neuroradiol. 2011;32(5):821–7.
Chappell KE, Robson MD, Stonebridge-Foster A, et al. Magic angle effects in MR neurography. Am J Neuroradiol. 2004;25(3):431–40.
Bendszus M, Koltzenburg M, Wessig C, Solymosi L. Sequential MR imaging of denervated muscle: experimental study. Am J Neuroradiol. 2002;23(8):1427–31.
Marciniak C. Practice parameter: utility of electrodiagnostic techniques in evaluating patients with suspected peroneal neuropathy: an evidence based review. Muscle Nerve. 2005;31:520–7.
Sourkes M, Stewart JD. Common peroneal neuropathy: a study of selective motor and sensory involvement. Neurology. 1991;41(7):1029–33.
Stewart JD. Foot drop: where, why and what to do? Pract Neurol. 2008;8(3):158–69.
Rha DW, Yi KH, Park ES, Park C, Kim HJ. Intramuscular nerve distribution of the hamstring muscles: application to treating spasticity. Clin Anat. 2016;29(6):746–51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Daniels, Dr. Ross, Dr. Gardon, and Ms. Li have no conflicts of interest.
Dr. Sneag discloses that HSS has a research agreement with GE.
Dr. Hanna receives royalties from Springer and Thieme as a book author.
Dr. Tuite receives royalties from Elsevier as a book author.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Daniels, S.P., Ross, A.B., Sneag, D.B. et al. Intravenous contrast does not improve detection of nerve lesions or active muscle denervation changes in MR neurography of the common peroneal nerve. Skeletal Radiol 50, 2483–2494 (2021). https://doi.org/10.1007/s00256-021-03812-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-021-03812-w