Abstract
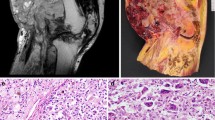
Diffuse-type tenosynovial giant cell tumor (TSGCT) is a rare, locally aggressive neoplasm. It most commonly occurs in the knee, followed by the hip, and has distinctive imaging features, including mass-like foci of low T2 signal intensity, “blooming” on gradient-echo MRI, and pronounced uptake on FDG PET/CT. Histologically, TSGCT demonstrates a neoplastic population of mononuclear cells admixed with hemosiderin-laden macrophages, foamy histiocytes, inflammatory cells, and osteoclast-like giant cells. In cases where diffuse-type TSGCT presents in an uncommon location or with atypical features, the imaging diagnosis may be challenging. Furthermore, because of its polymorphous appearance, it may be mistaken microscopically for other neoplastic and non-neoplastic histiocytic lesions. Herein, we present two cases of diffuse-type TSGCT presenting as large masses, and underscore the importance of radiologic-pathologic correlation for accurate diagnosis.




Similar content being viewed by others
References
Ottaviani S, Ayral X, Dougados M, Gossec L. Pigmented villonodular synovitis: a retrospective single-center study of 122 cases and review of the literature. Semin Arthritis Rheum. 2011;40(6):539–46.
Stevenson J, Jaiswal A, Gregory J, Mangham D, Cribb G, Cool P. Diffuse pigmented villonodular synovitis (diffuse-type giant cell tumour) of the foot and ankle. Bone Joint J. 2013;95(3):384–90.
Jaffe HL. Pigmented villonodular synovitis, bursitis, and tenosynovitis. Arch Pathol. 1941;31:731–65.
Mastboom MJ, Hoek DM, Bovée JV, van de Sande MA, Szuhai K. Does CSF 1 overexpression or rearrangement influence biological behaviour in tenosynovial giant cell tumours of the knee? Histopathology. 2019;74(2):332–40.
West RB, Rubin BP, Miller MA, Subramanian S, Kaygusuz G, Montgomery K, et al. A landscape effect in tenosynovial giant-cell tumor from activation of CSF1 expression by a translocation in a minority of tumor cells. Proc Natl Acad Sci. 2006;103(3):690–5.
Organization WH, Cancer IAfRo. WHO classification of tumours of soft tissue and bone. Tumours of soft tissue: introduction. Geneva: WHO; 2013. p. 281–95.
Murphey MD, Rhee JH, Lewis RB, Fanburg-Smith JC, Flemming DJ, Walker EA. Pigmented villonodular synovitis: radiologic-pathologic correlation. Radiographics. 2008;28(5):1493–518.
Verspoor FG, van der Geest IC, Vegt E, Veth RP, van der Graaf WT, Schreuder HW. Pigmented villonodular synovitis: current concepts about diagnosis and management. Future Oncol. 2013;9(10):1515–31.
Cheng XG, You YH, Liu W, Zhao T, Qu H. MRI features of pigmented villonodular synovitis (PVNS). Clin Rheumatol. 2004;23(1):31–4.
Masih S, Antebi A. Imaging of pigmented villonodular synovitis. Semin Musculoskelet Radiol. 2003;7(3):205–16.
Kang GH, Chi JG, Choi IH. Pigmented villonodular synovitis in the sacral joint with extensive bone destruction in a child. Pediatr Pathol. 1992;12(5):725–30.
Melamed A, Bauer CA, Johnson JH. Iliopsoas bursal extension of arthritic disease of the hip. Radiology. 1967;89(1):54–8.
Emile J-F, Diamond EL, Hélias-Rodzewicz Z, Cohen-Aubart F, Charlotte F, Hyman DM, et al. Recurrent RAS and PIK3CA mutations in Erdheim-Chester disease. Blood. 2014;124(19):3016–9.
Garner HW, Bestic JM. Benign synovial tumors and proliferative processes. Semin Musculoskelet Radiol. 2013;17(2):177–8.
Frick MA, Wenger DE, Adkins M. MR imaging of synovial disorders of the knee: an update. Radiol Clin N Am. 2007;45(6):1017–31 vii.
Broski SM, Murdoch NM, Skinner JA, Wenger DE. Pigmented villonodular synovitis: potential pitfall on oncologic 18F-FDG PET/CT. Clin Nucl Med. 2016;41(1):e24–31.
Eustace S, Goldberg R, Williamson D, Melhem E, Oladipo O, Yucel E, et al. MR imaging of soft tissues adjacent to orthopaedic hardware: techniques to minimize susceptibility artefact. Clin Radiol. 1997;52(8):589–94.
Young JR, Johnson GB, Murphy RC, Go RS, Broski SM. (18)F-FDG PET/CT in Erdheim-Chester disease: imaging findings and potential BRAF mutation biomarker. J Nucl Med. 2018;59(5):774–9.
Haroche J, Cohen-Aubart F, Emile J-F, Maksud P, Drier A, Tolédano D, et al. Reproducible and sustained efficacy of targeted therapy with vemurafenib in patients with BRAFV600E-mutated Erdheim-Chester disease. J Clin Oncol. 2014;33(5):411–8.
Arnaud L, Malek Z, Archambaud F, Kas A, Toledano D, Drier A, et al. 18F-fluorodeoxyglucose–positron emission tomography scanning is more useful in followup than in the initial assessment of patients with Erdheim-Chester disease. Arthritis Rheum. 2009;60(10):3128–38.
Sakamoto A, Matsuyama A, Hisaoka M, Matsuda S. Bone involvement mimicking an aggressive bone lesion in a diffuse-type tenosynovial giant cell tumor in the thoracic vertebral lamina: a case report. J Orthop Case Reports. 2018;8(3):14.
Dion E, Graef C, Miquel A, Haroche J, Wechsler B, Amoura Z, et al. Bone involvement in Erdheim-Chester disease: imaging findings including periostitis and partial epiphyseal involvement. Radiology. 2006;238(2):632–9.
Antunes C, Graca B, Donato P. Thoracic, abdominal and musculoskeletal involvement in Erdheim-Chester disease: CT, MR and PET imaging findings. Insights Imaging. 2014;5(4):473–82.
Diamond EL, Dagna L, Hyman DM, Cavalli G, Janku F, Estrada-Veras J, et al. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood. 2014;124(4):483–92.
Geldyyev A, Koleganova N, Piecha G, Sueltmann H, Finis K, Ruschaupt M, et al. High expression level of bone degrading proteins as a possible inducer of osteolytic features in pigmented villonodular synovitis. Cancer Lett. 2007;255(2):275–83.
Arnaud L, Hervier B, Neel A, Hamidou MA, Kahn JE, Wechsler B, et al. CNS involvement and treatment with interferon-alpha are independent prognostic factors in Erdheim-Chester disease: a multicenter survival analysis of 53 patients. Blood. 2011;117(10):2778–82.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
The need for informed consent was waived by the Institutional Review Board.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dundar, A., Young, J.R., Wenger, D.E. et al. Unusual manifestations of diffuse-type tenosynovial giant cell tumor in two patients: importance of radiologic-pathologic correlation. Skeletal Radiol 49, 483–489 (2020). https://doi.org/10.1007/s00256-019-03325-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-019-03325-7