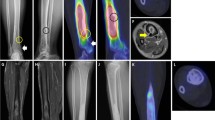
Abstract
Objective
To compare the healing pattern of osteoid osteomas on computed tomography (CT) and magnetic resonance imaging (MRI) after successful and unsuccessful thermocoagulation.
Materials and methods
Eighty-six patients were examined by CT and 18 patients by dynamic gadolinium-enhanced MRI before and after thermocoagulation for osteoid osteoma. Thermocoagulation was successful in 73% (63/86) and unsuccessful in 27% (23/86) of patients followed by CT. Thermocoagulation was successful in 72% (13/18) of patients followed by MRI. After treatment, the healing of the nidus on CT was evaluated using different healing patterns (complete ossification, minimal nidus rest, decreased size, unchanged size or thermonecrosis). On MRI the presence of reactive changes (joint effusion, “oedema-like” changes of bone marrow and soft tissue oedema) and the delay time (between arterial and nidus enhancement) were assessed and compared before and after thermocoagulation.
Results
Complete ossification or a minimal nidus rest was observed on CT in 58% (16/28) of treatment successes (with > 12 months follow-up), but not in treatment failures. “Oedema-like” changes of bone marrow and/or soft tissue oedema were seen on MR in all patients before thermocoagulation and in all treatment failures. However, residual “oedema-like” changes of bone marrow were also found in 69% (9/13) of treatment successes. An increased delay time was observed in 62% (8/13) of treatment successes and in 1/5 of treatment failures.
Conclusion
Complete, or almost complete, ossification of the treated nidus on CT correlated with successful treatment. Absence of this ossification pattern, however, did not correlate with treatment failure. CT could not be used to identify the activity of the nidus following treatment. The value of MR parameters to assess residual activity of the nidus was limited in this study.
Similar content being viewed by others
Introduction
Thermocoagulation is an effective and safe treatment for osteoid osteoma [1, 2]. The typical clinical and imaging features of osteoid osteoma on plain radiographs, bone scintigraphy and computed tomography (CT) have been described in detail [3–5]. The radiolucent nidus is best seen on thin slice (1 mm to 2 mm) axial CT images, because there is no overlap of surrounding reactive sclerosis and/or periosteal reaction [3–5]. More recent papers have focused on the appearance of osteoid osteoma on magnetic resonance imaging (MRI), describing the morphology of the nidus, the surrounding bone, and the adjacent soft tissues, as well as the enhancement pattern of the nidus during dynamic MRI imaging [6, 7].
The healing pattern of the nidus of osteoid osteomas has been described by Lindner et al. [8] and Martel et al. [9]. Since these imaging features have been reviewed in only successfully treated patients, it is unclear whether the imaging features during follow-up correlate with the clinical results of the thermocoagulation. The purpose of this study was, therefore, to compare the healing pattern of osteoid osteomas on CT and MRI in patients after successful and unsuccessful thermocoagulation.
Materials and methods
Patients
The records of 110 consecutive patients diagnosed with osteoid osteoma and treated with thermocoagulation between June 1994 and April 2000 were retrieved from our database. All patients gave informed consent for imaging studies and treatment. Our local institutional review board does not require informed consent for retrospective analysis of clinical data. Thirteen patients were excluded from this analysis because of incomplete follow-up data. Four of these 13 patients were excluded because of incomplete follow-up data, and nine of 13 patients were excluded because of a short (fewer than 3 months) but symptom-free follow-up. In the nine patients with fewer than 3 months’ follow-up, no further follow-up data after 3 months were available. Of these 110 patients, 97 had complete clinical follow-up data. In these 97 patients a total of 121 thermocoagulation procedures were performed. A treatment failure was defined as the presence of residual symptoms persisting at least 2 weeks after thermocoagulation or recurrence of symptoms resembling the initial symptoms (pain and/or impaired function). Otherwise the treatment was considered successful. In 76% (74/97) of these patients treatment was successful, and in 24% (23/97) of patients treatment failed after one thermocoagulation session. After the initial thermocoagulation procedure (97 procedures), 22/23 treatment failure patients underwent a second procedure. After this second procedure, two treatment failure patients underwent an additional third thermocoagulation session (one of these patients had recurrence of pain after 44 months) and two other patients had subsequent surgery. Thus, a total of 121 thermocoagulation procedures was performed in our patient group of 97 patients. The clinical outcome, and definitions of diagnosis and outcome, have been previously reported [1]. Anatomical location is listed in Table 1. Of these 97 patients 51% (49/97) had osteoid osteomas that were located extra-articularly and 49% (48/97) intra-articularly. As to the location of the nidus within bone, 65% (63/97) were cortical lesions, 23% (22/97) endosteal lesions, 10% (10/97) were intramedullary lesions and, in 2% (2/97), the location relative to the cortex was not documented.
As part of the initial diagnostic work-up of osteoid osteoma prior to thermocoagulation, 50/97 patients underwent MRI, 59/97 patients underwent scintigraphy, 94/97 patients had radiographs performed and all patients underwent CT scanning. After the radiological diagnosis was made using plain radiography, CT, bone-scintigraphy and MRI, CT-guided thermocoagulation was performed under regional or general anaesthesia by a radiologist and/or orthopaedic surgeon. The nidus was localised using incremental CT (Tomoscan CXQ or LX; Philips Medical Systems, Best, The Netherlands) or helical CT (Tomoscan SR 7000 or AV E1; Philips Medical Systems).
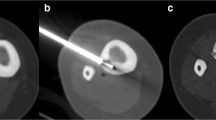
Through a small skin incision, the centre of the lesion was engaged, initially by using a Steinmann pin (Synthes, Bettlach, Switzerland) during the first 40 procedures and later by using a 14-gauge “Bonopty” needle system (Radi Medical Systems, Uppsala, Sweden) during the other 81 procedures. A biopsy (including cultures) was performed if the clinical or radiographic appearance of the lesion was atypical, in clinical practice mainly to rule out a Brodie’s abscess. If a biopsy were needed, a needle system (Jamshidi; Sherwood Medical, Belfast, Northern Ireland) was introduced over the K wire of the Steinmann pin, or the “Bonopty” needle system drill was removed and exchanged for a 16-gauge biopsy needle. The position of the biopsy needle was always monitored with CT. After the biopsy needle had been removed, a dedicated cannula and, subsequently, a radio-frequency probe [Sluijter-Metha cannula (20-gauge, length 145 mm, active tip 5 mm) for radiofrequency probe, and a radiofrequency probe, both by Radionics, Burlington, Massachusetts, USA] were introduced through the penetration cannula.
The temperature at the tip of the thermocoagulation electrode was monitored during the procedure. The lesions were routinely heated to 90°C for 4 min (RFG-3C FF lesion generator system, Radionics). After removal of the needle system a CT scan was performed to assess where the nidus had been hit and to check for possible complications (e.g. haematoma). CT was able to determine whether the nidus had been hit, since the needle track was visualised after the procedure.
The mean clinical follow-up time after the only or last thermocoagulation session was 41 months (range 5–81 months).
CT follow-up
According to our clinical protocol, all patients should have had clinical, CT and MRI follow-up at 3 months, 6 months, 12 months and 24 months after thermocoagulation. This succeeded quite well for the clinical follow-up, but was moderately successful for the CT follow-up and not very successful for the MRI follow-up in these patients. The reason was that the orthopaedic surgeon taking care of these patients adhered quite strictly to the clinical portion but not as strictly to the imaging portion of the follow-up protocol.
Eighty-nine percent (86/97) of patients were followed by CT scan after undergoing thermocoagulation. This was after the only thermocoagulation performed in successfully treated patients and after the first or only thermocoagulation procedure performed in treatment-failure patients (one of 23 failure patients had only one thermocoagulation session).
The mean CT follow-up time in these 86 patients was 14 months, range 2–32 months. If patients had more than one follow-up CT scan performed after thermocoagulation, then the last follow-up CT was used to evaluate bone healing. For instance, if a patient had a follow-up CT at 6 months and 12 months, this patient was considered to have a CT follow-up of 12 months, and this follow-up CT 12 months after thermocoagulation was used to evaluate this bone healing. Thermocoagulation treatment was successful in 63 patients (73%). In 27% (23/86) of patients thermocoagulation did not result in a significant clinical improvement. The 63 successfully treated patients had a mean CT follow-up period of 15 months (range 6–32 months) after the only thermocoagulation performed, and the 23 treatment failures had a mean CT follow-up time of 10 months (range 2–28 months) after the first or only thermocoagulation performed (as mentioned before, one of 23 treatment-failure patients had only one thermocoagulation procedure). The mean clinical follow-up after the only or last thermocoagulation for this group of 86 patients was 44 months (range 6–81 months). In all patients the duration of the clinical follow-up was at least as long as the duration of the CT follow-up.
CT follow-up examination was performed by incremental or helical CT scanning. Incremental CT scanning was performed with a 1–2 mm slice-by-slice thickness (Tomoscan CXQ or LX; Philips Medical Systems). Helical CT scanning was performed with a reconstructed slice thickness of 1 mm to 2 mm (Tomoscan SR 7000, or AV E1; Philips Medical Systems).
A nidus was identified in all cases on a CT scan before thermocoagulation. We compared follow-up CT scans with the pre-treatment one, and classified changes into one of five groups defined as: (1) complete ossification of the nidus, (2) presence of a minimal nidus rest, (3) decrease in size of the nidus, (4) unchanged size of the nidus, and (5) changed configuration of the nidus, the latter most likely being related to osteonecrosis after thermocoagulation.
MRI follow-up
According to our clinical protocol, all patients should have had clinical, CT and MRI follow-up at 3 months, 6 months, 12 months and 24 months after thermocoagulation. As mentioned before, this succeeded quite well for the clinical follow-up, but was not as successful for the CT and MRI follow-up in these patients. In addition, a relatively low number of patients was included for MRI follow-up compared with CT follow-up. This is explained by the fact that progressively, during the period between June 1994 and April 2000, only patients with clinical suspicion of treatment failure were selected for further MRI follow-up.
In 18 selected cases a gadolinium-enhanced dynamic MRI was performed during follow-up (mean 12 months, range 2–28 months) after thermocoagulation. This was after the only thermocoagulation performed in successfully treated patients and after the first or only thermocoagulation procedure performed in treatment-failure patients (one of five treatment-failure patients had only one thermocoagulation session). As for CT scanning, if patients had undergone more than one follow-up MRI, then the last follow-up MRI was used to assess eventual changes in “oedema-like” alterations of bone marrow and/or soft tissue oedema and joint effusion as well as the delay time. The 13 successfully treated patients in this group had a mean MRI follow-up period of 14 months (range 3–28 months) after the only thermocoagulation, and the five treatment-failure patients had a mean MRI follow-up period of 5 months (range 2–10 months) after the first or only thermocoagulation (as mentioned before, one of the five treatment-failure patients included had only one thermocoagulation session). The mean clinical follow-up time after the only or last thermocoagulation in these 18 patients was 33 months (range 6–81 months). In all patients the duration of the clinical follow-up was at least as long as the duration of the MRI follow-up.
A non-contrast coronal T1-turbo spin echo (TSE) sequence (TR 550 ms, TE 12 ms), a transverse T1 gradient echo sequence (T1 FFE) (TR 26 ms, TE 8.6 ms) and a transverse T2 TSE sequence with fat suppression (TR 2,980 ms, TE 80 ms) were performed (Gyroscan ACS NT 15, Philips Medical Systems). For dynamic imaging, three consecutive sections were taken through the lesion in the transverse plane. A bolus injection of gadopentate dimeglumine (Gd-DTPA) was administered (0.1 mmol/kilogramme body weight, injected at 2 ml/s). A gradient echo sequence was used for the dynamic sequence (T1 FFE, TR 9.5 ms, TE 3 ms) with a temporal resolution of 3 s per image for a total of 120 s. All contrast images were subtracted from the first non-enhanced gradient echo image, and the time of enhancement of the osteoid osteoma was related to the arterial enhancement (delay time). An equilibrium contrast-enhanced series was obtained, consisting of a T1 FFE with fat suppression (TR 45 ms, TE 8.6 ms). All sequences were performed with a 256 pixel × 256 pixel matrix. As for CT scanning, the nidus was demonstrable on all MRI studies before thermocoagulation was carried out.
Two parameters were assessed and compared on the MRI studies performed before and after thermocoagulation: (1) The presence of “oedema-like” changes of bone marrow and/or soft tissue oedema or joint effusion; (2) the delay time between the onset of arterial enhancement and enhancement of the nidus, which was assessed by dynamic MRI.
Analysis and statistical correlation
All CT scans and MR images were read and interpreted in consensus by two musculoskeletal radiologists (G.V. and another radiologist). The analysing radiologists were not aware of the clinical data.
Prior to statistical analysis the five CT categories were grouped into two main categories: (A). advanced bone healing (complete ossification, and minimal residual nidus) and (B) minimal or absent bone healing (decreased size, and unchanged size of the nidus). Further statistical analysis of bone healing on CT was carried out relative to the treatment outcome (treatment success versus failure), the length of CT follow-up, the location of the nidus within bone (cortical versus an endosteal or an intramedullary location) and the patient’s age. Because of the limited number of patients in the group with thermonecrosis, this group was not included in this statistical analysis.
The chi-square test and Fisher’s exact test were used to evaluate differences in the fraction of patients with advanced bone healing on CT between successfully treated patients and patients with treatment failure. To test the effect of the length of CT follow-up on bone healing, we performed the same statistical tests on two subgroups with a CT follow-up period of 2–12 months, and > 12 months, respectively. To test for differences in bone healing relative to the location of the nidus within bone (cortical versus an endosteal or an intramedullary location), we used the chi-square test, and to test for differences in age between groups with different healing patterns we used the t-test. A P value < 0.05 was considered statistically significant. A software package (SPSS 12.0.1; SPPS, Chicago, Illinois, USA) was used for statistical analysis. The number of patients in the MRI follow-up was too small for an adequate statistical analysis to be performed.
Results
Histology
A biopsy was taken in 58% (56/97) of patients. Histology confirmed the presence of an osteoid osteoma in 36% (20/56) of patients. In the remainder a definite histological diagnosis could not be made, because the amount of biopsy material was too small. The diagnosis of osteoid osteoma was confirmed histologically in 22% (19/86) of patients in the CT follow-up group and in 22% (4/18) of patients in the MRI follow-up group.
CT follow-up
Table 2 shows the distribution of the healing pattern on CT in patients with successful and unsuccessful thermocoagulation. Complete ossification, a minimal nidus remnant or thermonecrosis was seen only in patients whose treatment had been successful (Table 2, Figs. 1 and 2). Advanced bone healing was more frequently (P < 0.001) observed in the successfully treated group (29/63 patients or 46%) than in the treatment failure group (0 patients). Interestingly, all 15 patients (listed in Table 2) with complete ossification of the nidus had osteoid osteomas that were located within the cortex, and, in patients with an endosteal or an intramedullary location of the nidus, the highest degree of bone healing observed was the presence of a minimal nidus rest, but not complete ossification. This relationship was statistically significant (P = 0.03).
Table 3 illustrates the distribution of the healing pattern for the two subgroups with 2–12 months or > 12 months of CT follow-up. Advanced bone healing (the presence of complete ossification and/or a minimal nidus rest) was more frequently seen in the group with > 12 months’ follow-up (58% or 16/28 patients) than in the group with a shorter follow-up period (37% or 13/35 patients), but this difference in bone healing between the two groups was not statistically significant (P = 0.07).
No statistically significant relationship between bone healing and age (in successfully and unsuccessfully treated patients) was present, both in patients with a shorter (2–12 months) and a longer (> 12 months) CT follow-up period. The P value mentioned hereafter was observed in the successfully treated group with a > 12 months CT follow-up (P = 0.1).
MRI follow-up
Of the 18 patients who were followed with MRI, treatment was successful in 13 (72%). Because of the limited number of patients included in this MRI follow-up group, no adequate statistical analysis could be performed, as has been mentioned previously.
Table 4 shows the distribution of associated reactive bone and soft tissue changes before and after thermocoagulation on MRI in patients with successful and unsuccessful thermocoagulation. All patients had shown “oedema-like” changes of bone marrow and/or soft tissue edema or joint effusion before thermocoagulation.
After treatment, three successfully treated patients showed complete resolution of these oedematous or “oedema-like” changes (Fig. 3). These three patients had exhibited no joint effusion prior to thermocoagulation, and, thus, no residual changes (“oedema-like” changes of bone marrow, soft tissue oedema or joint effusion) were present after thermocoagulation. A fourth patient in whom these oedematous or “oedema-like” changes had completely resolved had shown persistent joint effusion after successful thermocoagulation. The other nine of 13 treatment successes (69%) had residual “oedema-like” changes of bone marrow, while all five treatment failures had residual “oedema-like” changes of bone marrow (two of these patients also had residual soft tissue oedema).
Axial gadolinium-enhanced T1 FFE (TR 45 ms, TE 8.6 ms) with fat suppression of the left hip before (a) and 24 months after (b) successful thermocoagulation. Before thermocoagulation, marked enhancement of the nidus (arrow) and the surrounding bone marrow and soft tissues was identified. No residual enhancement in and around the nidus was seen after (successful) thermocoagulation. The corresponding CT scan (not shown) 24 months after treatment demonstrated a decreased diameter of the nidus, but no complete ossification
None of the patients with treatment failure showed significant change in the pattern of “oedema-like” changes of bone marrow or soft tissue oedema after thermocoagulation (Fig. 4). Complete resolution was thus not observed in this group.
Axial gadolinium-enhanced T1 FFE (TR 45 ms, TE 8.6 ms) with fat suppression of the left hip before (a) and 3 months after (b) unsuccessful thermocoagulation. Before thermocoagulation, marked enhancement of the nidus (arrow) and the surrounding bone marrow and soft tissues was identified, persisting at 3 months. On dynamic gadolinium-enhanced MRI (not shown) rapid enhancement of the nidus (with a delay time of 4 s) persisted after (unsuccessful) thermocoagulation
Before thermocoagulation, joint effusion was seen in 44% (8/18) of patients (Table 4). All lesions in these eight patients had an intra-articular location (three were located in the proximal femur, three in the talus and two in the pelvis). This joint effusion persisted in all patients after thermocoagulation, independent of treatment outcome (five were treatment successes, the other three treatment failures).
Table 5 shows the delay time before and after thermocoagulation on dynamic gadolinium-enhanced MRI in patients with successful and unsuccessful thermocoagulation. The results suggest that an increase in delay time tends to be associated with successful treatment, although the number of patients is small. Before thermocoagulation, all osteoid osteomas showed a rapid enhancement within 12 s after arterial enhancement. In the successfully treated patient group eight of the 13 patients (62%) showed either slow (> 12 s delay time) or no enhancement. In the treatment-failure group only one of the five patients showed enhancement after 12 s.
Discussion
During CT follow-up there was a significant difference in the appearance of the osteoid osteomas between patients with successful and unsuccessful thermocoagulation. Complete, or almost complete, ossification of the nidus was seen only in patients (46% of good respondents) who were successfully treated. CT findings, however, were of limited value, because absence of this ossification pattern occurred equally in patients with good and poor response to treatment. Ossification occurs mainly within the first year of treatment, as we did not find significant progression of ossification after the first year.
These results are similar to the results published by Lindner et al. [8], who found complete ossification of the nidus in 53% (8/15) of successfully treated patients. In their study the CT follow-up time was limited to 6 months, and no results of unsuccessfully treated patients were reported. Lindner et al. [8] also reported that osteoid osteomas located within the cortex had a greater tendency to ossify after successful thermocoagulation than those in other intra-osseous locations. We made a similar observation after successful thermocoagulation: complete ossification of the nidus (present in 15 patients, Table 2) was exclusively seen in patients with a cortical location of the nidus, and this relationship was statistically significant (P = 0.03).
A larger fraction of complete ossification of 75% (24/32 patients) was found in a study by Martel et al. [9] in successfully treated patients after 12 months of follow-up with CT scan. The higher percentage of complete ossification in the latter study may be related to the thermocoagulation technique that was applied. In our study a technique was used with a non-cooled tip and an active tip length of 5 mm, identical to the technique that was used by Lindner et al. [8]. From a theoretical perspective [4], we know that this type of electrode creates an approximately 1 cm spherical treatment zone of focal osteonecrosis. Martel et al. [9] used a cooled electrode with an active tip of 10 mm. The treatment zone in this technique is expected to be larger, due to the longer size of the active tip, and the infusion of saline, allowing greater heat transmission [4]. This larger treatment zone and thus larger area of thermal injury may have caused more inflammation and secondary ossification and might, therefore, have contributed to a higher degree of ossification. Because of this larger treatment zone, the use of a cooled tip is generally not advocated for thermocoagulation of osteoid osteomas, because of safety considerations. This applies especially to osteoid osteomas of the spinal canal, because of the risk of thermal injury to the spinal cord or adjacent neurovascular structures [10, 11].
Other authors have also commented on the presence of bone healing on CT scans after previous thermocoagulation. Pinto et al. [4] mentioned that partial or complete ossification of the nidus was to be expected over 2–27 months, although little or no change in lesion appearance was possible. In some cases the nidus could become indistinguishable from surrounding bone, and reactive changes in adjacent bone and periosteum tended to diminish. Similar observations were made by Ghanem et al. [12] in a series of 12 patients with osteoid osteomas that were successfully treated with thermocoagulation. The lowest percentage of complete ossification after successful thermocoagulation of osteoid osteoma was reported by Papagelopoulos et al. [13]. They treated 16 intra-articular osteomas of the hip region with a 10 mm active but non-cooled tip. At 12 months’ CT follow-up, 19 % (3/16) of osteoid osteomas treated in their series demonstrated complete ossification; the other lesions demonstrated partial ossification (six patients) or no change (seven patients).
Martel et al. [9] reported that at a CT follow-up time of 12 months, all successfully treated patients without obvious bone healing were over 16 years of age. In our study, however, no statistically significant correlation between bone healing on CT and the patient’s age was identified.
The presence of reactive changes in association with osteoid osteoma has been described before [6]. Osteoid osteomas produce a large amount of prostaglandins. Elevations of the prostaglandin levels up to 100–1,000 times the normal level have been demonstrated within the nidus of osteoid osteomas [6]. The production of such high levels of prostaglandins generates inflammation of the peri-tumoral soft tissue, causing reactive changes (synovitis and/or effusion, and “oedema-like” changes of bone marrow as well as soft tissue oedema) [6].
On MRI, all patients showed “oedema-like” changes of bone marrow and/or soft tissue oedema before they had undergone thermocoagulation or when the treatment had failed (Table 4). However, residual “oedema-like” changes of bone marrow were found in 69% (9/13) of treatment successes. From a theoretical point of view, one would expect a total absence of “oedema-like” changes of bone marrow (or soft tissue oedema) after successful thermocoagulation, reflecting the total heat-induced destruction of the hormonally active nidus [6]. It is unclear why these “oedema-like” changes of bone marrow persist after successful thermocoagulation. It may be that pain decreases because of the destruction of nerve fibres in and around the nidus [14] after successful thermocoagulation, while hormone production is maintained. Other possible explanations include local mechanical or degenerative changes [6], or reaction to the procedure; in this latter case one would expect these “oedema-like” changes of bone marrow to diminish gradually or disappear after successful thermocoagulation. Further study is required to determine the exact nature and evolution of these residual “oedema-like” changes of bone marrow present in successfully treated patients.
Joint effusion, present before thermocoagulation, persisted in all of our patients after thermocoagulation, independent of the treatment outcome. Consequently, because this joint effusion persisted in all patients, it was not valuable in differentiating treatment successes from treatment failures in our series. It is likely that the underlying mechanism is the same as for the persistent “oedema-like” changes of bone marrow and soft tissue oedema described above.
The pattern of dynamic enhancement of non-treated osteoid osteomas on MRI has been studied in detail by Liu et al. [7]. They assessed the morphology or type of enhancement curve, and found that nine of 11 patients (82%) had peak enhancement of the osteoid osteoma in the early arterial phase with early partial wash-out. In our study we focused on the delay time (time between the onset of arterial and nidus enhancement). Before treatment, all 18 osteoid osteomas showed enhancement within 12 s of the onset of arterial enhancement (Table 5).
Although absence of enhancement was not observed in the group with failed treatment, only 38% (5/13) of patients with good response showed absence of enhancement (Table 5). Only one patient with poor response had delayed enhancement (> 12 s), while 62% (8/13) of successfully treated patients showed no, or delayed, enhancement. Slower enhancement may reflect the replacement of highly vascular tissue by less vascularised or even avascular tissue (such as scar tissue, fibrosis or bone) after successful thermocoagulation. Although small numbers do not allow statistical analysis, it seems that the low incidence of change in enhancement indicates low potential for this parameter to be useful in clinical practice.
Although the role of skeletal scintigraphy in the primary diagnosis of osteoid osteoma has been reported in detail previously [15], its use in the detection of residual or recurrent osteoid osteoma after surgery or thermocoagulation has not received much attention. Some case reports [16, 17] have indicated, though, that scintigraphy may reliably identify persistent or recurrent osteoid osteoma in a post-operative setting.
Conclusion
Minimal or even absent bone healing of the nidus did not necessarily indicate treatment failure, since it was also observed in successfully treated patients. Therefore, based upon CT findings solely, we could not differentiate between treatment successes and failures. The value of MRI in assessing the residual activity of the nidus after thermocoagulation was limited in this study. Clinical evaluation remains essential in identifying patients that may benefit from repeated thermocoagulation.
References
Vanderschueren GM, Taminiau AH, Obermann WR, Bloem JL. Osteoid osteoma: clinical results with thermocoagulation. Radiology 2002; 224(1): 82–86.
Vanderschueren GM, Taminiau AH, Obermann WR, van den Berg-Huysmans AA, Bloem JL. Osteoid osteoma: factors for increased risk of unsuccessful thermal coagulation. Radiology 2004; 233(3): 757–762.
Greenspan A. Benign bone-forming lesions: osteoma, osteoid osteoma, and osteoblastoma. Clinical, imaging, pathologic, and differential considerations. Skeletal Radiol 1993; 22(7): 485–500.
Pinto CH, Taminiau AH, Vanderschueren GM, Hogendoorn PC, Bloem JL, Obermann WR. Technical considerations in CT-guided radiofrequency thermal ablation of osteoid osteoma: tricks of the trade. AJR Am J Roentgenol 2002; 179(6): 1633–1642.
Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am 1998; 80(6): 815–821.
Gaeta M, Minutoli F, Pandolfo I, Vinci S, D’Andrea L, Blandino A. Magnetic resonance imaging findings of osteoid osteoma of the proximal femur. Eur Radiol 2004; 14(9): 1582–1589.
Liu PT, Chivers FS, Roberts CC, Schultz CJ, Beauchamp CP. Imaging of osteoid osteoma with dynamic gadolinium-enhanced MR imaging. Radiology 2003; 227(3): 691–700.
Lindner NJ, Ozaki T, Roedl R, Gosheger G, Winkelmann W, Wortler K. Percutaneous radiofrequency ablation in osteoid osteoma. J Bone Joint Surg Br 2001; 83(3): 391–396.
Martel J, Bueno A, Ortiz E. Percutaneous radiofrequency treatment of osteoid osteoma using cool-tip electrodes. Eur J Radiol 2005; 56(3): 403–408.
Bitsch RG, Rupp R, Bernd L, Ludwig K. Osteoid osteoma in an ex vivo animal model: temperature changes in surrounding soft tissue during CT-guided radiofrequency ablation. Radiology 2006; 238(1): 107–112.
Dupuy DE, Hong R, Oliver B, Goldberg SN. Radiofrequency ablation of spinal tumors: temperature distribution in the spinal canal. AJR Am J Roentgenol 2000; 175(5): 1263–1266.
Ghanem I, Collet LM, Kharrat K, Samaha E, Deramon H, Mertl P, et al. Percutaneous radiofrequency coagulation of osteoid osteoma in children and adolescents. J Pediatr Orthop B 2003; 12(4): 244–252.
Papagelopoulos PJ, Mavrogenis AF, Kyriakopoulos CK, Benetos IS, Kelekis NL, Andreou J, et al. Radiofrequency ablation of intra-articular osteoid osteoma of the hip. J Int Med Res 2006; 34(5): 537–544.
O’Connell JX, Nanthakumar SS, Nielsen GP, Rosenberg AE. Osteoid osteoma: the uniquely innervated bone tumor. Mod Pathol 1998; 11(2): 175–180.
Helms CA, Hattner RS, Vogler JB III. Osteoid osteoma: radionuclide diagnosis. Radiology 1984; 151(3): 779–784.
Groshar D, Alperson M, Liberson A. Bone scintigraphy in recurrent osteoid osteoma of the talus. Clin Nucl Med 1996; 21(10): 817–818.
Kumar R, Chandrashekhar N, Dasan JB, et al. Recurrent osteoid osteoma: a case report with imaging features. Clin Imaging 2003; 27(4): 269–272.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Vanderschueren, G.M., Taminiau, A.H.M., Obermann, W.R. et al. The healing pattern of osteoid osteomas on computed tomography and magnetic resonance imaging after thermocoagulation. Skeletal Radiol 36, 813–821 (2007). https://doi.org/10.1007/s00256-007-0319-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-007-0319-1