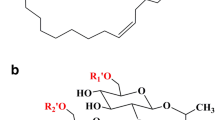
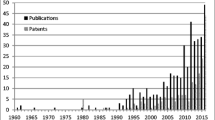
Abstract
Sophorolipids (SLs), mainly synthesized by yeasts, were a sort of biosurfactant with the highest fermentation level at present. In recent years, SLs have drawn extensive attention for their excellent physiochemical properties and physiological activities. Besides, issues such as economics, sustainability, and use of renewable resources also stimulate the shift from chemical surfactants towards green or microbial-derived biosurfactants. SLs’ large-scale production and application were restricted by the relatively high production costs. Currently, waste streams from agriculture, food and oil refining industries, etc., have been exploited as low-cost renewable substrates for SL production. Advanced cultivation method, uncommonly used substrates, and new genetically modified SL-producing mutants were also designed and applied to improve the productivity or the special properties of SLs. In this review, a systematic and detailed description of primary and secondary metabolism pathways involved in SL biosynthesis was summarized firstly. Furthermore, based on the pathways of SL biosynthesis from different carbon substrates, we reviewed the current knowledge and advances in the exploration of cost-effective and infrequently used hydrophilic and hydrophobic substrates for large or specialized SL production.


Similar content being viewed by others
References
Achlesh D, Kannan P (2009) Production, characterization, and properties of sophorolipids from the yeast Candida bombicola using a low-cost fermentative medium. Appl Biochem Biotechnol 158:663–774
Achlesh D, Kannan P (2010a) Kinetics of growth and enhanced sophorolipids production by Candida bombicola using a low-cost fermentative medium. Appl Biochem Biotechnol 160:2090–2101
Achlesh D, Kannan P (2010b) Sophorolipids from Candida bombicola using mixed hydrophilic substrates: production, purification and characterization. Colloids Surf B Biointerfaces 79:246–253
Ashby RD, Dky S, Foglia TA (2006) The use of fatty acid esters to enhance free acid sophorolipid synthesis. Biotechnol Lett 28:253–260
Asmer HJ, Lang S, Wagner F, Wray V (1988) Microbial production, structure elucidation and bioconversion of sophorose lipids. J Am Oil Chem Soc 65:1460–1466
Bajaj VK, Annapure US (2015) Castor oil as secondary carbon source for production of sophorolipids using Starmerella bombicola NRRL Y-17069. J Oleo Sci 64:315–323
Bhangale A, Wadekar S, Kale S, Bhowmick D, Pratap A (2014) Production of sophorolipids synthesized on castor oil with glucose and glycerol by using Starmerella bombicola (ATCC 22214). Eur J Lipid Sci Technol 116:336–343
Brakemeier A, Lang S, Wullbrandt D, Merschel L, Benninghoven A, Buschmann N, Wagner F (1995) Novel sophorose lipids from microbial conversion of 2-alkanols. Biotechnol Lett 17:1183–1188
Brakemeier A, Wullbrandt D, Lang S (1998a) Candida bombicola: production of novel alkyl glycosides based on glucose/2-dodecanol. Appl Microbiol Biotechnol 50:161–166
Brakemeier A, Wullbrandt D, Lang S (1998b) Microbial alkyl-sophorosides based on 1-dodecanol or 2-, 3- or 4-dodecanones. Biotechnol Lett 20:215–218
Casas JA, Garcia-Ochoa F (1999) Sophorolipid production by Candida bombicola: medium composition and culture methods. J Biosci Bioeng 88:488–494
Cavalero DA, Cooper DG (2003) The effect of medium composition on the structure and physical state of sophorolipids produced by Candida bombicola ATCC 22214. J Biotechnol 103:31–41
Ciesielska K, Roelants SL, Van Bogaert IN, De Waele S, Vandenberghe I, Groeneboer S, Soetaert W, Devreese B (2016) Characterization of a novel enzyme Starmerella bombicola lactone esterase (SBLE) responsible for sophorolipid lactonization. Appl Microbiol Biotechnol 100:9529–9541
Cooper DG, Paddock DA (1984) Production of a biosurfactant from Torulopsis bombicola. Appl Environ Microbiol 47:173–176
Daniel HJ, Otto RT, Reuss M, Syldatk C (1998a) Sophorolipid production with high yields on whey concentrate and rapeseed oil without consumption of lactose. Biotechnol Lett 20:805–807
Daniel HJ, Reuss M, Syldatk C (1998b) Production of sophorolipids in high concentration from deproteinized whey and rapeseed oil in two stage fed-batch process using Candida bombicola ATCC 22214 and Cryptococcus curvatus ATCC 20509. Biotechnol Lett 20:1153–1156
Daniel HJ, Otto RT, Binder M, Reuss M, Syldatk C (1999) Production of sophorolipids from whey: development of a two-stage process with Cryptococcus curvatus ATCC 20509 and Candida bombicola ATCC 22214 using deproteinized whey concentrates as substrates. Appl Microbiol Biotechnol 51:40–45
Darne PA, Mehta MR, Agawane SB, Prabhune AA (2016) Bioavailability studies of curcumin–sophorolipid nano-conjugates in the aqueous phase: role in the synthesis of uniform gold nanoparticles. RSC Adv 6:68504–68514
Daverey A, Pakshirajan K (2009) Production of sophorolipids by the yeast Candida bombicola using simple and low cost fermentative media. Food Res Int 42:499–504
Davila AM, Marchal R, Vandecasteele JP (1992) Kinetics and balance of a fermentation free from product inhibition: sophorose lipid production by Candida bombicola. Appl Microbiol Biotechnol 38:6–11
Davila AM, Marchal R, Vandecasteele JP (1994) Sophorose lipid production from lipidic precursors: Predictive evaluation of industrial substrates. J Ind Microbiol Biot 13:249–257
Davila AM, Marchal R, Vandecasteele JP (1997) Sophorose lipid fermentation with differentiated substrate supply for growth and production phases. Appl Microbiol Biotechnol 47:496–501
Dengle-Pulate V, Chandorkar P, Bhagwat S, Prabhune AA (2014) Antimicrobial and SEM studies of sophorolipids synthesized using lauryl alcohol. J Surfactants Deterg 17:543–552
Esders TW, Light RJ (1972) Glucosyl- and acetyltransferases involved in the biosynthesis of glycolipids from Candida bogoriensis. J Biol Chem 247:1375–1386
Felse PA, Shah V, Chan J, Rao KJ, Gross RA (2007) Sophorolipid biosynthesis by Candida bombicola from industrial fatty acid residues. Enzyme Microb Technol 40:316–323
Fleurackers SJJ (2006) On the use of waste frying oil in the synthesis of sophorolipids. Eur J Lipid Sci Technol 108:5–12
Fleurackers SJJ, Bogaert INAV, Develter D (2010) On the production and identification of medium-chained sophorolipids. Eur J Lipid Sci Technol 112:655–662
Göbbert U, Lang S, Wagner F (1984) Sophorose lipid formation by resting cells of Torulopsis bombicola. Biotechnol Lett 6:225–230
Guilmanov V, Ballistreri A, Impallomeni G, Gross RA (2002) Oxygen transfer rate and sophorose lipid production by Candida bombicola. Biotechnol Bioeng 77:489–494
Gupta R, Prabhune AA (2012) Structural determination and chemical esterification of the sophorolipids produced by Candida bombicola grown on glucose and alpha-linolenic acid. Biotechnol Lett 34:701–707
Hommel RK, Weber L, Weiss A, Himmelreich U, Rilke O, Kleber HP (1994) Production of sophorose lipid by Candida (Torulopsis) apicola grown on glucose. J Biotechnol 33:147–155
Huaimin W, Roelants S, To M, Patria R, Kaur G, Sze Lau N, Yin Lau C, Van Bogaert I, Soetaert W, Lin C (2018) Starmerella bombicola: recent advances on sophorolipid production and prospects of waste stream utilization. J Chem Technol Biotechnol 94:999–1007
Imura T, Kawamura D, Morita T, Sato S, Fukuoka T, Yamagata Y, Takahashi M, Wada K, Kitamoto D (2013) Production of sophorolipids from non-edible jatropha oil by Stamerella bombicola NBRC 10243 and evaluation of their interfacial properties. J Oleo Sci 62:857–864
Jiménez-Peñalver P, Gea T, Sánchez A, Font X (2016) Production of sophorolipids from winterization oil cake by solid-state fermentation: Optimization, monitoring and effect of mixing. Biochem Eng J 115:93–100
Jiménez-Peñalver P, Castillejos M, Koh A, Gross R, Sánchez A, Font X, Gea T (2018) Production and characterization of sophorolipids from stearic acid by solid-state fermentation, a cleaner alternative to chemical surfactants. J Clean Prod 172:S0959652617328111
Jones DF (1968) Microbiological oxidation of long-chain aliphatic compounds. V. Mechanism of hydroxylation. J Chem Soc Perkin Trans 22:2827–2833
Joshi-Navare K, Khanvilkar P, Prabhune A (2013) Jatropha oil derived sophorolipids: production and characterization as laundry detergent additive. Biochem Res Int 2013:169797
Kim SY, Oh DK, Lee KH, Kim JH (1997) Effect of soybean oil and glucose on sophorose lipid fermentation by Torulopsis bombicola in continuous culture. Appl Microbiol Biotechnol 48:23–26
Kim HS, Kim YB, Lee BS, Kim EK (2005) Sophorolipid production by Candida bombicola ATCC 22214 from a corn-oil processing byproduct. J Microbiol Biotechnol 15:55–58
Kim YB, Yun HS, Kim EK (2009) Enhanced sophorolipid production by feeding-rate-controlled fed-batch culture. Bioresour Technol 100:6028–6032
Klekner V, Kosaric N, Zhou QH (1991) Sophorose lipids produced from sucrose. Biotechnol Lett 13:345–348
Koganti S (2012) Conversion of biodiesel byproduct glycerol to arabitol and sophorolipids through microbial fermentation. University of Akron, Dissertation
Konishi M, Morita T, Fukuoka T, Imura T, Uemura S, Iwabuchi H, Kitamoto D (2018) Efficient production of acid-form sophorolipids from waste glycerol and fatty acid methyl esters by Candida floricola. J Oleo Sci 67:489–496
Lee KH, Kim JH (1993) Distribution of substrates carbon in sophorose lipid production by Torulopsis bombicola. Biotechnol Lett 15:263–266
Li H, Ma XJ, Wang S, Song X (2013) Production of sophorolipids with eicosapentaenoic acid and docosahexaenoic acid from Wickerhamiella domercqiae var. sophorolipid using fish oil as a hydrophobic carbon source. Biotechnol Lett 35:901–908
Liu XG, Ma XJ, Yao RS, Pan CY, He HB (2016) Sophorolipids production from rice straw via SO3 micro-thermal explosion by Wickerhamiella domercqiae var. sophorolipid CGMCC 1576. AMB Express 6:1–11
Ma XJ, Li H, Wang DX, Song X (2014) Sophorolipid production from delignined corncob residue by Wickerhamiella domercqiae var. sophorolipid CGMCC 1576 and Cryptococcus curvatus ATCC 96219. Appl Microbiol Biotechnol 98:475–483
Maddikeri GL, Gogate PR, Pandit AB (2015) Improved synthesis of sophorolipids from waste cooking oil using fed batch approach in the presence of ultrasound. Chem Eng Sci 263:479–487
Makkar RS, Cameotra SS, Banat IM (2011) Advances in utilization of renewable substrates for biosurfactant production. AMB Express 1:1–19
Makoto T, Tomotake M, Koji W, Naoto H, Tokuma F, Tomohiro I, Dai K (2011) Production of sophorolipid glycolipid biosurfactants from sugarcane molasses using Starmerella bombicola NBRC 10243. J Oleo Sci 60:267–273
Maneerat S (2005) Production of biosurfactants using substrates from renewable resources. J Sci and Tech 27:675–683
Masaaki K, Yuka Y, Jun-Ichi H (2015) Efficient production of sophorolipids by Starmerella bombicola using a corncob hydrolysate medium. J Biosci Bioeng 119:317–322
Minucelli T, Ribeiro-Viana RM, Borsato D, Andrade G, Cely MVT, Oliveira MRD, Baldo C (2016) Sophorolipids production by Candida bombicola ATCC 22214 and its potential application in soil bioremediation. Waste & Biomass Valorization 8:1–11
Nuñez A, Ashby RA, Foglia T, Solaiman D (2001) Analysis and characterization of sophorolipids by liquid chromatography with atmospheric pressure chemical ionization. Chromatographia 53:673–677
Oliveira MRD, Camilios-Neto D, Baldo C, Magri A, Celligoi MAPC (2014) Biosynthesis and production of sophorolipids. Int J Sci Tech Res 3:133–146
Oliveira MRD, Magri A, Baldo C, Camilios-Neto D, Minucelli T, Celligoi MAPC (2015) Minireview: sophorolipids a promising biosurfactant and its applications. Int J Adv Biotechnol Res 6:161–174
Parekh VJ, Patravale VB, Pandit A (2012) Mango kernel fat: A novel lipid source for the fermentative production of sophorolipid biosurfactant using Starmerella bombicola NRRL-Y 17069. Ann Biol Res 3:1798–1803
Pekin G, Vardar-Sukan F, Kosaric N (2005) Production of sophorolipids from Candida bombicola ATCC 22214 using Turkish corn oil and honey. Eng Life Sci 5:357–362
Rashad MM, Al-kashef AS, Nooman MU, Mahmoud AEE (2014a) Engco-utilization of motor oil waste and sunflower oil cake on the production of new sophorolipids by Candida bombicola NRRL-Y 17069. Res J Pharm Biol Chem Sci 5:1515–1528
Rashad MM, Nooman MU, Ali MM, Al-kashef AS, Mahmoud AE (2014b) Production, characterization and anticancer activity of Candida bombicola sophorolipids by means of solid state fermentation of sunflower oil cake and soybean oil. Grasas Aceites 65:e017
Rau U, Manzke C, Wagner F (1996) Influence of substrate supply on the production of sophorose lipids by Candida bombicola ATCC 22214. Biotechnol Lett 18:149–154
Rau U, Hammen S, Heckmann R, Wray V, Lang S (2001) Sophorolipids: a source for novel compounds. Ind Crops Prod 13:85–92
Saerens KM, Roelants SL, Van Bogaert IN, Soetaert W (2011a) Identification of the UDP-glucosyltransferase gene UGTA1, responsible for the first glucosylation step in the sophorolipid biosynthetic pathway of Candida bombicola ATCC 22214. FEMS Yeast Res 11:123–132
Saerens KM, Saey L, Soetaert W (2011b) One-step production of unacetylated sophorolipids by an acetyltransferase negative Candida bombicola. Biotechnol Bioeng 108:2923–2931
Saerens KM, Zhang J, Saey L, Van Bogaert IN, Soetaert W (2011c) Cloning and functional characterization of the UDP-glucosyltransferase UgtB1 involved in sophorolipid production by Candida bombicola and creation of a glucolipid-producing yeast strain. Yeast 28:279–292
Saerens KM, Van Bogaert IN, Soetaert W (2015) Characterization of sophorolipid biosynthetic enzymes from Starmerella bombicola. FEMS Yeast Res 15:1–9
Saharan B, Sahu R, Sharma D (2011) A review on biosurfactants: fermentation, current developments and perspectives. Genet Eng Biotechnol J 29:1–39
Samad A (2015) Sophorolipid production from lignocellulosic biomass feedstocks. Southern Illinois University Carbondale, Dissertation
Samad A, Zhang J, Chen D, Liang Y (2015) Sophorolipid production from biomass hydrolysates. Appl Biochem Biotechnol 175:2246–2257
Samad A, Zhang J, Chen D, Chen X, Tucker M, Liang Y (2017) Sweet sorghum bagasse and corn stover serving as substrates for producing sophorolipids. J Ind Microbiol Biotechnol 44:353–362
Shah V, Doncel GF, Seyoum T, Eaton KM, Zalenskaya I, Hagver R, Azim A, Gross R (2005) Sophorolipids, microbial glycolipids with anti-human immunodeficiency virus and sperm-immobilizing activities. Antimicrob Agents Chemother 49:4093–4100
Shah V, Jurjevic M, Badia D (2007) Utilization of restaurant waste oil as a precursor for sophorolipid production. Biotechnol Prog 23:512–515
Shah MUH, Sivapragasam M, Moniruzzaman M, Talukder MMR, Yusup SB, Goto M (2017) Production of sophorolipids by Starmerella bombicola yeast using new hydrophobic substrates. Biochem Eng J 127:60–67
Solaiman DKY (2005) Sophorolipid biosynthesis from biodiesel co-product stream. J Am Oil Chem Soc 82:625–630
Solaiman DKY, Ashby RD, Nuñez A, Foglia TA (2004) Production of sophorolipids by Candida bombicola grown on soy molasses as substrate. Biotechnol Lett 26:1241–1245
Solaiman DKY, Ashby RD, Zerkowski JA, Foglia TA (2007) Simplified soy molasses-based medium for reduced-cost production of sophorolipids by Candida bombicola. Biotechnol Lett 29:1341–1347
Takahashi F, Igarashi K, Hagihara H (2016) Identification of the fatty alcohol oxidase FAO1 from Starmerella bombicola and improved novel glycolipids production in an FAO1 knockout mutant. Appl Microbiol Biotechnol 100:9519–9528
Tulloch AP, Spencer JFT, Gorin PAJ (1962) The fermentation of long-chain compounds by Torulopsis magnoliae. I. Structures of the hydroxy fatty acids obtained by the fermentation of fatty acids and hydrocarbons. Can J Chem 40:1326–1338
Van Bogaert IN, Saerens K, De Muynck C, Develter D, Soetaert W, Vandamme EJ (2007) Microbial production and application of sophorolipids. Appl Microbiol Biotechnol 76:23–34
Van Bogaert IN, Develter D, Soetaert W, Vandamme EJ (2008) Cerulenin inhibits de novo sophorolipid synthesis of Candida bombicola. Biotechnol Lett 30:1829–1832
Van Bogaert IN, Roelants S, Develter D, Soetaert W (2010a) Sophorolipid production by Candida bombicola on oils with a special fatty acid composition and their consequences on cell viability. Biotechnol Lett 32:1509–1514
Van Bogaert IN, Sabirova J, Develter D, Soetaert W, Vandamme EJ (2010b) Knocking out the MFE-2 gene of Candida bombicola leads to improved medium-chain sophorolipid production. FEMS Yeast Res 9:610–617
Van Bogaert IN, Fleurackers S, Van Kerrebroeck S, Develter D, Soetaert W (2011a) Production of new-to-nature sophorolipids by cultivating the yeast Candida bombicola on unconventional hydrophobic substrates. Biotechnol Bioeng 108:734–741
Van Bogaert IN, Zhang J, Soetaert W (2011b) Microbial synthesis of sophorolipids. Process Biochem 46:821–833
Van Bogaert IN, Holvoet K, Roelants S, Li B, Lin YC, Van de Peer Y, Soetaert W (2013) The biosynthetic gene cluster for sophorolipids: a biotechnological interesting biosurfactant produced by Starmerella bombicola. Mol Microbiol 88:501–509
Vatsa P, Sanchez L, Clement C, Baillieul F, Dorey S (2010) Rhamnolipid biosurfactants as new players in animal and plant defense against microbes. Int J Mol Sci 11:5095–5108
Vaughn SF, Behle RW, Skory CD, Kurtzman CP, Price NPJ (2014) Utilization of sophorolipids as biosurfactants for postemergence herbicides. Crop Prot 59:29–34
Vedaraman N, Venkatesh N (2010) The effect of medium composition on the production of sophorolipids and the tensiometric properties by Starmerella bombicola MTCC 1910. Pol J Chem Technol 12:9–13
Wadekar SD, Kale SB, Lali AM, Bhowmick DN, Pratap AP (2012a) Sophorolipid production by Starmerella bombicola (ATCC 22214) from virgin and waste frying oils, and the effects of activated earth treatment of the waste oils. J Am Oil Chem Soc 89:1029–1039
Wadekar SD, Kale SB, Lali AM, Bhowmick DN, Pratap AP (2012b) Jatropha oil and karanja oil as carbon sources for production of sophorolipids. Eur J Lipid Sci Technol 114:823–832
Wadekar SD, Kale SB, Lali AM, Bhowmick DN, Pratap AP (2012c) Utilization of sweetwater as a cost-effective carbon source for sophorolipids production by Starmerella bombicola (ATCC 22214). Prep Biochem Biotechnol 42:125–142
Waele S, Vandenberghe I, Laukens B, Planckaert S, Verweire S, Van Bogaert IN, Soetaert W, Devreese B, Ciesielska K (2018) Optimized expression of the Starmerella bombicola lactone esterase in Pichia pastoris through temperature adaptation, codon-optimization and co-expression with HAC1. Protein Expr Purif 143:62–70
Zhou QH (1995) Utilization of canola oil and lactose to produce biosurfactant with Candida bombicola. J Am Oil Chem Soc 72:67–71
Zhou QH, Kosaric N (1993) Effect of lactose and olive oil on intra- and extracellular lipids of Torulopsis bombicola. Biotechnol Lett 15:477–482
Funding
This work was supported by the Fundamental Research Funds for the Central Universities of China (No. JZ2019YYPY0029), the National Natural Science Foundation of China (No. 31400049), and the China Postdoctoral Science Foundation (No. 2015T80646).
Author information
Authors and Affiliations
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, X., Meng, L., Zhang, H. et al. Sophorolipid biosynthesis and production from diverse hydrophilic and hydrophobic carbon substrates. Appl Microbiol Biotechnol 104, 77–100 (2020). https://doi.org/10.1007/s00253-019-10247-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10247-w