Abstract
Non-dairy milk alternatives (or milk analogues) are water extracts of plants and have become increasingly popular for human nutrition. Over the years, the global market for these products has become a multi-billion dollar business and will reach a value of approximately 26 billion USD within the next 5 years. Moreover, many consumers demand plant-based milk alternatives for sustainability, health-related, lifestyle and dietary reasons, resulting in an abundance of products based on nuts, seeds or beans. Unfortunately, plant-based milk alternatives are often nutritionally unbalanced, and their flavour profiles limit their acceptance. With the goal of producing more valuable and tasty products, fermentation can help to the improve sensory profiles, nutritional properties, texture and microbial safety of plant-based milk alternatives so that the amendment with additional ingredients, often perceived as artificial, can be avoided. To date, plant-based milk fermentation mainly uses mono-cultures of microbes, such as lactic acid bacteria, bacilli and yeasts, for this purpose. More recently, new concepts have proposed mixed-culture fermentations with two or more microbial species. These approaches promise synergistic effects to enhance the fermentation process and improve the quality of the final products. Here, we review the plant-based milk market, including nutritional, sensory and manufacturing aspects. In addition, we provide an overview of the state-of-the-art fermentation of plant materials using mono- and mixed-cultures. Due to the rapid progress in this field, we can expect well-balanced and naturally fermented plant-based milk alternatives in the coming years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant-based milk alternatives have been consumed for hundreds and thousands of years. These products are meant to resemble animal milk, an emulsion containing nutrients such as lipids, proteins, amino acids, vitamins and minerals and produced by lactating mammals to provide nutrients for the growth and development of their sucklings (Haug et al. 2007; Mäkinen et al. 2016; Sethi et al. 2016). Today, milk alternatives are commercially obtained from a variety of plants, such as legumes, seeds, nuts, cereals and pseudo-cereals (Mäkinen et al. 2016). Over the past years, the market for these plant-based milk alternatives has continually increased and, in the USA alone, reached an annual volume of approximately US$1.8 billion (Fig. 1). From a global perspective, the projected compound annual growth rate (CAGR) is higher than 10%, and thus the world market is estimated to surpass US$26 billion by 2023 (Bloomberg Surveillance 2015). The increasing preference for plant-based milk alternatives is driven by different factors and consumer demands: health-related challenges such as lactose intolerance and milk allergies (Crittenden and Bennett 2005), consumer concerns about cow milk hormones and cholesterol (Epstein 1990), ethical disputes regarding the use of animals (Hughes 1995), environmental issues (Rotz et al. 2010), changes in lifestyle towards vegetarian and vegan food, presumably healthier diet (Craig 2010) and the marketed health-promoting properties of these products (Paucar-Menacho et al. 2010). Accordingly, leading dairy companies are adding plant-based milk alternative products to their portfolio.
U.S. market development for plant-based milk alternative products (soy, almond and other non-dairy milk products). The data are taken from Bloomberg Surveillance (2015)
Plant-based milk alternatives are intended to resemble animal milk in terms of colour and texture (Mäkinen et al. 2016; Sethi et al. 2016). However, they often do not provide the full nutritional value of cow milk (Sethi et al. 2016) and suffer from undesirable off-flavours (Desai et al. 2002; Sethi et al. 2016; Vanga and Raghavan 2018). Therefore, commercial products positioned as plant-based milk alternatives are typically amended with additives such as vitamins, amino acids, and minerals (Sethi et al. 2016). However, leading food and beverage companies have committed to removing ingredients perceived as artificial from their products—clean label foods and beverages are not only a trend but are seemingly becoming an expectation. Accordingly, natural plant-based milk alternatives, which meet the nutritional quality and taste of animal-derived milk without blending, are of particular interest (Asioli et al. 2017).
An appealing option to reach this goal is fermentation. Since the early days of mankind, fermentation has been a natural approach to produce food, and today, fermented foods are more popular than ever before (Adler et al. 2013). During the production of coffee, bread, chocolate, wine, cheese, mixed pickles, kombucha, kimchi, and sauerkraut, food-grade microbes improve the nutritional value, aroma and taste, texture and stability of foods and beverages and contribute to their microbial safety. In particular, the application of mono-culture fermentation to food products is well understood (Leroy and De Vuyst 2004; National Research Council 1992). More recently, the design of mixed-culture fermentation with two or more microorganisms, naturally occurring in many food production processes (Adler et al. 2013), is becoming increasingly important (Ciani et al. 2010; Smid and Lacroix 2013). The latter appears particularly promising for plant-based milk alternative fermentation due to the potential synergistic effects within the microbial consortia, which helps to improve quite diverse quality criteria with only one process (National Research Council 1992; Sieuwerts et al. 2008).
In this regard, this review introduces the key criteria for major plant-based milk alternatives, including nutritional and sensory qualities as well as manufacturing perspectives. In relevant examples, mono- and mixed-culture fermentations of plant-based milk alternatives are presented to highlight state-of-the-art and future avenues for research and development.
Leading plant-based milk alternatives
Plant types
Due to a constantly increasing demand for non-dairy alternatives and growing interest in exploring different functional properties, various plants have been used to produce non-dairy milk alternatives (Sethi et al. 2016). The relevant plant sources can be classified into five types: (i) legumes (beans), (ii) nuts, (iii) seeds, (iv) pseudo-cereals, and (v) cereals (Fig. 3) (Sethi et al. 2016). Soy-based drinks are the dominant plant-based milk alternatives in the Western world (Mäkinen et al. 2016). In addition, drinks based on almond (Ginsberg and Ostrowski 2007), coconut (Seow and Gwee 1997), sunflower seed (Fujisawa et al. 1986), chickpea (Rao et al. 1988), lupine (Ivanović et al. 1983), hemp (Vahanvaty 2009), sesame (Afaneh et al. 2011), quinoa (Pineli et al. 2015), pea (Li et al. 2004), and rice (Mitchell et al. 1990) are available and contribute to the diversity of the plant-based milk alternative market. Depending on the individual raw materials, the corresponding drinks differ significantly in composition and flavour.
Key quality criteria
Plant-based milk alternatives should preferably resemble the technical, nutritional and organoleptic properties of cow milk. To achieve this goal, researchers and developers in academia and industry must overcome certain challenges (Figs. 2 and 3).
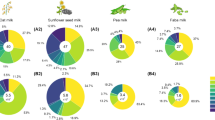
Macronutrient composition, functional components and limiting factors of common plants used for plant-based milk alternative production. The data are collected from previous work (Afaneh et al. 2011; Bernat et al. 2015; Callaway 2004; DebMandal and Mandal 2011; Duranti et al. 2008; Erbaş et al. 2005; Fernandez and Berry 1988; Hove 1974; Juliano and Hicks 1996; Lambo et al. 2005; Lampart-Szczapa et al. 2003; Lebiedzińska and Szefer 2006; Makinde and Akinoso 2013; Moneret-Vautrin et al. 1999; Noimark and Cox 2008; Önning et al. 1998; Paucar-Menacho et al. 2010; Ranhotra et al. 1993; Roy et al. 2010; Seow and Gwee 1997; Sethi et al. 2016; Škrbić and Filipčev 2008; Ulyatu et al. 2015; Vahanvaty 2009; Vanga and Raghavan 2018; Vidal-Valverde et al. 2003; Vilche et al. 2003; Villamide and San Juan 1998; Wood and Grusak 2007). The micronutrient composition data are acquired from the National Nutrient Database for Standard Reference Release (NDB) (https://ndb.nal.usda.gov/ndb/). The NDB identification of the selected materials is as follows: milk (01212), soy (16111), chickpea (45041830), pea (45272128), lupine (16076), coconut milk (45117929), almond (12061), sunflower seed kernels (12036), hemp seed (12012), sesame seed (12023), quinoa (20035), rice (20090) and oat (20132). carb, carbohydrates except fibre; funct. peptides, functional peptides; unsaturated FA, unsaturated fatty acids.
Physico-chemical properties
Plant-based milk alternative manufacturing generally employs consecutive unit operations (Fig. 4). Generally, plant-based drinks are prepared by crushing the plant material, followed by extraction of its soluble parts into water. The properties of the final product depend on the raw material and, furthermore, on the specifications of the individual disintegration, homogenisation, formulation, emulsification, and storage processes. Different strategies are applied to make the homogenisation and stability of plant-based milks more similar to that of animal milk, which is a natural emulsion. For example, plant-based drinks from starchy materials (such as cereals or pseudo-cereals) easily gelate during sterilisation (autoclaving or pasteurisation), which causes technical problems in downstream processing (Mäkinen et al. 2016). Furthermore, the excessive lipid content of seeds and nuts may lead to an undesired phase separation and reduced product stability (Figs. 2 and 3) so that these compounds are removed during processing (Briviba et al. 2016). More details on plant-based milk alternative manufacturing can be found in an excellent recent review (Mäkinen et al. 2016).
Nutritional value and bioactive components
Without doubt, the plants used offer certain attractive properties (Fig. 3). Some of the raw materials, such as legumes and seeds, have a protein content comparable to that of cow milk (although the amino acid quality is not comparable to the same extent). Moreover, the raw materials are rich in certain micronutrients (vitamin, minerals) (Gernand et al. 2016) and contain bioactive compounds such as antioxidants (Zhao and Shah 2014), dietary fibres (Kohajdová et al. 2006) and phytoestrogens (Verdeal and Ryan 1979) (Figs. 2 and 3). The latter contributes to health benefits, including a lowered risk of osteoporosis, heart disease, breast cancer, and menopausal symptoms (Patisaul and Jefferson 2010). However, phytohormones potentially also cause adverse health effects, which may depend on human age, health status and even the presence or absence of specific gut microflora, so that this area requires more research in the future (Patisaul and Jefferson 2010).
In recent years, several interesting studies have investigated the presence and impact of micro-nutrients and bioactive compounds in plant materials. As an example, almond, peanut, and coconut exhibit significant amounts of vitamins E and C, which confer antioxidant properties (Sethi et al. 2016). Legumes are a good source for essential mono- and polyunsaturated fatty acids, minerals (Fe2+, Zn2+, Mg2+) (Sandberg 2002), and phytoestrogens (isoflavones) (Pyo et al. 2005) (Figs. 2 and 3). In other types of plant materials, β-glucans contribute to health benefits (in lowering cholesterol levels) and increase the sensory attributes of the final products (Lazaridou and Biliaderis 2007; Othman et al. 2011).
Despite these undoubted beneficial properties of plant raw materials, a careful inspection, however, reveals that commercial plant-based milk alternatives are not nutritionally balanced and comparable to animal milk. In particular, the protein content of plant-based drinks can be low. Approximately 50% of commercial plant-based milk alternatives contain little or even no protein (< 0.5%), while only selected soy-based milk analogues reach the higher protein level of cow milk (3.7%) (Jeske et al. 2017). Additionally, plant proteins often exhibit low quality, poor digestibility and an undesired limitation in essential amino acids (Millward 1999). l-Lysine, l-methionine, l-cysteine and l-tryptophan are amino acids that are typically underrepresented (Millward 1999). In addition, certain vitamins, such as vitamin D and vitamin B12, are present at low levels or are even absent (Table 1), which may be part of the reason for the vitamin deficiency of people following a strict vegetarian diet (Pawlak et al. 2014). Moreover, vitamins are sensitive molecules and some of them are easily degraded during washing and heating, which further reduces their content (Fig. 4). Other important compounds suffer from low bioavailability. For instance, soy isoflavones mainly exist in the form of genistin and daidzin, which are glucosides of genistein and daidzein and far less bioavailable than the corresponding aglycone forms (Vacek et al. 2008; Xu et al. 1994). Moreover, plant-derived products can contain anti-nutritional factors. For example, phytates and saponins form insoluble complexes with valuable minerals (such as Ca2+, Mg2+, Fe2+ and Zn2+), which decrease their bioavailability (Rekha and Vijayalakshmi 2010; West et al. 1978). Plant-based oligosaccharides, such as raffinose, stachyose, and verbascose, can only be digested by intestinal bacteria through fermentation, which results in flatulence, diarrhoea, and other discomforts (Onyesom et al. 2005). Furthermore, the intestinal tract can be disturbed by trypsin and other protease inhibitors in plant-based milk alternatives, which interfere with protein and starch digestion by inactivating the digesting enzymes (Anderson and Wolf 1995).
Sensory profile
It has been shown by consumer and marketing studies that taste has a key impact on food selection (Glanz et al. 1998). In this regard, the natural taste of plant-based milk alternatives, unfortunately, exhibits only limited acceptance (Mäkinen et al. 2016). Although certain components of plant materials (such as soluble fibres) positively influence texture and mouthfeel (Vasquez-Orejarena et al. 2018), plant-based milk alternatives are still generally perceived as products with a displeasing taste, probably also because of previous experiences with less appealing products in the market (Wansink et al. 2005). Legume-based products tend to smell beany and earthy, which is considered undesirable in countries without traditional consumption of these types of products. Volatile compounds such as n-hexanal and n-hexanol, which originate from the oxidation of plant lipids, are mainly responsible for this type of off-flavour. Plant phenols (including anti-nutrients such as tannins and saponins), terpenes, glucosinolates, and flavonoids impart bitter, acrid or astringent tastes, depending on their molecular weights (Drewnowski and Gomez-Carneros 2000). Regrettably, certain bioactive (and therefore otherwise beneficial) compounds such as isoflavonoids are also linked to an objectionable aftertaste (Matsuura et al. 1989). Additionally, a greenish, greyish or brownish colour, which corresponds to the colour of the raw plant material; a chalky or sandy texture; and a thin mouthfeel due to the presence of insoluble particles negatively influence consumer purchase willingness (Peyer et al. 2016).
Technical processing and fortification
To solve some of the abovementioned challenges, different manufacturing strategies have been developed. In early processing steps, excess lipids (from nuts and seeds) and excess starch (from cereals and pseudo-cereals) are separated and/or enzymatically hydrolysed to prevent phase separation and gelation and increase product stability (Rustom et al. 1993). Homogenisation is used to disrupt larger particles and lipid droplets and achieve uniform particle size, which also improves product stability (Briviba et al. 2016).
To overcome the known nutritional and sensory limitations, commercial plant-based milk alternatives are typically supplemented with sweeteners, artificial flavours, protein, amino acids, minerals (Ca2+, Mg2+, Fe2+ and Zn2+), and vitamins (B12, B2, D and E) (Sethi et al. 2016; Zhang et al. 2007). Moreover, extended mechanical and thermal pre-processing (e.g. roasting, dehulling, blanching, soaking, cooking and sprouting) is applied to reduce anti-nutrients such as protease inhibitors (Jiang et al. 2013; Yuan et al. 2008), decrease and mask off-flavour and improve mouthfeel and colour (Dakwa et al. 2005; Kim et al. 1986). However, some anti-nutrients are very resistant. For example, phytates cannot be destroyed entirely even by heating to 100 °C (Anderson and Wolf 1995).
Fermentation of plant materials
Fermentation has been applied to cereals such as maize, wheat, rice and sorghum for a long time (National Research Council 1992). Plant materials support the growth of microorganisms (Espirito-Santo et al. 2014; Peyer et al. 2016; Sethi et al. 2016). Lactic acid bacteria (LAB), bacilli and yeasts (e.g. Saccharomyces) are the most widely used microbes for plant-based fermentation (Jeske et al. 2018; Steinkraus 1997). Being studied mainly as mono-cultures, these microbes have been proven to possess properties that enhance important nutritional and/or sensory attributes.
Nutritional value
Most importantly, fermentation can increase protein content by the growth of the fermenting food-grade microbes and by improving plant protein solubility and amino acid composition and availability. As an example, Bifidobacterium significantly increased the crude protein content of soy-based drinks (Hou et al. 2000). Moreover, fermentation of soybean meal with Lactobacillus plantarum resulted in a beneficial increase in essential amino acids such as l-lysine (Song et al. 2008). Notably, specific microbial strains synthesise vitamins during fermentation (LeBlanc et al. 2013), including vitamin K (Bentley and Meganathan 1982) and vitamins of the B group (LeBlanc et al. 2011). Yeast is well known for its ability to produce vitamin B2 (Lindegren 1945). Compared to synthetic fortification, fortification by natural vitamin-producing microorganisms is widely recognised as safer, more natural and more environmentally friendly (LeBlanc et al. 2011).
Anti-nutrients and mineral availability
Fermentation by itself or combined with other treatments such as cooking, sprouting and soaking can dramatically reduce the level of anti-nutrients such as tannins, phytates and cyanides in plant-based food (Anderson and Wolf 1995; Onyesom et al. 2005; Soetan and Oyewole 2009; Wang et al. 2003). As an example, LAB are capable of producing phytases and provide the optimum pH conditions for these enzymes, which then catalyse the hydrolysis of phytates into myo-inositol and phosphate, improve digestibility and increase mineral bioavailability (Rekha and Vijayalakshmi 2010). As an example, fermentation of finger millet significantly reduced various undesired anti-nutrients (phytates, tannins, and trypsin inhibitor) while simultaneously enhancing mineral extractability and digestibility (Antony and Chandra 1998).
Bioactive components
Fermentation is capable of increasing the concentration or bioaccessibility of functional (bioactive) compounds. The fermentation of soy using bacteria with β-glucosidase ability enables the conversion of glucoside isoflavones into aglycone isoflavones of higher bioactivity and bioaccessibility (Pyo et al. 2005), which has also been observed for seeds of kerandang, a flowering plant belonging to the legume family (Titiek et al. 2013). Correspondingly, L. plantarum is able to transform sesaminol triglucoside of sesame milk into bioactive sesaminol aglycone with enhanced radical scavenging activity (Ulyatu et al. 2015). It was also reported that LAB fermentation of soy releases bioactive peptides, which inhibit angiotensin-converting enzymes that are related to the desired antihypertensive effect (Hou et al. 2000).
Sensory profile
Fermentation can improve the sensory profile of plant-based milk alternatives (Mital and Steinkraus 1979). As an example, microbial fermentation decreased the beany flavour of plant materials, probably due to deprivation of n-hexanal and n-hexanol (Wang et al. 2003). In addition, fermentation can result in desirable volatile flavours. For example, diacetyl (2,3-butanedione), which provides a nice, butterscotch-like aroma, is emitted during cereal-based fermentation (Peyer et al. 2016). Acetaldehyde, delivering a pungent, fruity (green apple) flavour with sweet notes, increases in concentration in peanut, cereal and soy during fermentation (Horáčková et al. 2015; Lee and Beuchat 1991; Sethi et al. 2016). The flavour and taste of plant-based milk alternatives is also affected by changes in the levels of amino acids (Yamanaka et al. 1970).
Mixed-culture fermentation can provide synergistic effects to enhance quality
An interesting and obviously important concept is the use of mixed cultures to ferment plant materials. Generally, interactions between microbes in mixed cultures are numerous and complex, for example competition (−/− interaction), mutualism (+/+ interaction), commensalism (+/0 interaction), amensalism (−/0 interaction), and parasitism (+/− interaction) (Sieuwerts et al. 2008). Desired interactions during mixed-culture fermentation are mainly of a mutualistic and commensalistic nature, by which beneficial activities of at least one microbe are promoted (National Research Council 1992).
The cooperation between Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus during yogurt fermentation is a well-understood example of mutualism. The proteolytic Lactobacillus strain benefits the non-proteolytic S. thermophilus through the release of peptides and free amino acids as a nitrogen source. Conversely, S. thermophilus provides L. delbrueckii with growth-stimulating factors such as formic acid, pyruvic acid, folic acid and carbon dioxide (Sieuwerts et al. 2008). In a mixed culture, the two strains stimulate one another’s growth, acid production and volatile compound formation (Sieuwerts et al. 2008). A synergistic effect on growth has also been observed for soy fermentation (Chumchuere and Robinson 1999), probably related to the different glycolytic activities of the strains involved. More specifically, microbial consortia can cooperate to enable multistep biotransformations. A prominent example demonstrated a close cooperation of microbes in simulated cocoa pulp fermentation: LAB form lactate, while yeasts form ethanol during the early stage of the fermentation. Both nutrients, in turn, are crucial co-substrates for acetic acid bacteria, which then accumulate acetate, the key molecule that initiates the formation of aroma and flavour compounds (Adler et al. 2013). Demonstrated effects of mixed cultures in the generation of plant-based milk alternatives are summarised in Figs. 5 and 6 and are described in more detail in the following subsections.
Impact of mixed-culture fermentation on the quality of plant-based milk alternatives. Comparison between mixed-culture and mono-culture fermentation: microbial growth (A), essential amino acid level (B), vitamin level (C). The white area covers desired synergistic effects (desired), while the grey area covers undesired effects that result from mixed-culture fermentation (undesired)
Growth
During soy fermentation, Lactobacillus fermentum NRRC 207 and L. delbrueckii subsp. bulgaricus NCDO 1489 exhibited more than 100-fold higher cell numbers when mixed with S. thermophilus than when grown in mono-culture, and both strains improved the growth of S. thermophilus as well (Chumchuere and Robinson 1999). Such a synergetic effect was also observed in other studies (Champagne et al. 2009; Mital et al. 1974). The combination of amylolytic and probiotic bacterial strains also reduced the fermentation time of rice because of the resulting elevated acidification rate (Espirito-Santo et al. 2014). In addition, certain yeasts benefit the growth of LAB by excreting specific nutrients (Liu and Tsao 2009; Rekha and Vijayalakshmi 2010). However, not all combinations are desirable for the survival of starter cultures (Angelov et al. 2005). As an example, the viable count of Bifidobacterium longum R015 and L. fermentum even decreased in specific mixed-culture fermentation (Fig. 5a) (Champagne et al. 2009; Chumchuere and Robinson 1999).
Nutrient value
The protein content and essential amino acid composition can differ substantially following mono- vs. mixed-culture fermentation of plant-based milk alternatives (Fig. 5b). Co-fermentation of peanut using Lactobacillus acidophilus and L. plantarum significantly increased the total protein and l-lysine, l-methionine and l-tryptophan contents compared to those of the corresponding mono-culture fermentations (Sanni et al. 1999). Spontaneous co-fermentation of strains originating from cowpea and chickpea improved l-methionine levels by approximately sixfold (Zamora and Fields 1979). However, in other cases, mixed-culture fermentation appeared inferior to mono-culture processes (Santos et al. 2014).
Moreover, mixed cultures can impact vitamin formation. Co-fermentation of L. plantarum SM39 and Propionibacterium freudenreichii DF13 showed higher levels of folate and vitamin B12 and yielded up to 8400 ng/L of folate, which is, otherwise, only achievable with genetically modified strains (Hugenschmidt et al. 2011; Smid and Lacroix 2013). To date, only a few pioneering studies have investigated bacterial vitamin production during plant material fermentation (Fig. 5c). A mixture of Lactobacillus strains, including L. plantarum, improved the riboflavin, thiamine and niacin contents in cowpea milk compared to the use of mono-cultures (Sanni et al. 1999). Similar effects were observed for soy using a co-fermentation of Saccharomyces boulardii and Lactobacillus casei (Rekha and Vijayalakshmi 2010). However, many of the tested strain combinations only showed few or even adverse effects in this study (Champagne et al. 2010; Zamora and Fields 1979). It has been speculated that a decrease in a specific vitamin may relate to the fact that the organism itself requires it for growth (Rekha and Vijayalakshmi 2010).
Anti-nutrients and mineral availability
Interestingly, mixed cultures help to reduce anti-nutrients (Fig. 6a), which, in turn, enhances mineral availability (Fig. 6b). A mixed culture of L. acidophilus and L. plantarum was more effective than fermentation of the individual strains in eliminating phytic acid and trypsin inhibitors in cowpea (Sanni et al. 1999). Similarly, a mixed culture of S. thermophilus CCRC 14085 and Bifidobacterium infantis CCRC 14603 dramatically decreased phytic acid (80%) and saponin (30%) levels in soy (Lai et al. 2013). It was further found that a mixed S. boulardii and L. plantarum B4495 fermentation increased calcium bioavailability approximately sixfold compared to the mono-culture fermentation (Rekha and Vijayalakshmi 2010).
Stachyose and raffinose are undesirable components of plant-based milk alternatives, especially legume-based products, that are linked to flatulence (Desai et al. 2002). Generally, a combination of different strains was more efficient in degrading these carbohydrates than were pure cultures (Santos et al. 2014). Soy fermented by a mixed culture of different LAB yielded a lower level of stachyose and raffinose and a desirable higher content of acetic acid, fructose, glucose and galactose (Wang et al. 2003). Similar effects were also observed in other studies (Santos et al. 2014). However, these outcomes are strongly dependent on the exact strain combination. As an example, Streptococcus induced adverse effects in L. fermentum and Bifidobacterium longum to metabolise stachyose (Champagne et al. 2009; Chumchuere and Robinson 1999).
Bioactive components
Fermentation of soy by bacteria that possess β-glucosidase activity enables biotransformation of isoflavones into the more bioactive aglycone form (Pyo et al. 2005). Mixed cultures of S. boulardii together with five Lactobacillus species converted over 95% of the glucoside into the aglycone isoflavone (Rekha and Vijayalakshmi 2010). However, other strain mixtures revealed lower bioconversion efficiency compared to that of pure cultures. For example, a weaker bioconversion was detected when S. thermophilus was mixed with B. infantis, B. longum and Lactobacillus helveticus (Champagne et al. 2010; Chien et al. 2006). This observation emphasises again the importance of strain selection in mediating the synergetic effects between the different cultures.
Sensory values
Although mono-culture fermentation seems to be as efficient as mixed-culture fermentation in lowering the content of the off-flavour molecules n-hexanal and n-hexanol (Lee 2001), mixed-culture fermentation appears to be more useful in generating preferred flavour enhancers. For example, acetaldehyde, a key compound of the desired yogurt flavour, is formed more extensively by a mixture of two or more cultures (Horáčková et al. 2015; Lee 2001; Liu et al. 2002). A mixed culture of L. delbrueckii subsp. bulgaricus and Streptococcus salivarius subsp. thermophilus not only decreased the beany flavour of peanut milk but also significantly increased whiteness, viscosity, gumminess and smoothness (Lee 2001). An increase in luminosity and whiteness index values was also observed following almond fermentation by a mixed culture of Lactobacillus reuteri and S. thermophilus (Bernat et al. 2015).
Conclusion
The market for plant-based milk alternatives is quickly increasing. However, the unbalanced nutrition and unwanted organoleptic characteristics of these products still limit their consumption. The use of mixed-culture fermentation, in particular, holds great potential for improving the nutritional quality and the sensory profile of plant materials. Previous studies clearly show that the performance of mixed cultures is strongly species- and strain-dependent. At present, strain combination is still conducted with trial and error approaches. It seems difficult, if not even infeasible, to predict the effects of a mixed culture due to our still poor understanding of the underlying microbial interactions involved. Possibilities for a more rational selection and combination of strains with predictable synergistic interactions would be highly valuable towards developing smarter fermentation processes and better products.
Recently, systems biology approaches have greatly advanced and opened up novel possibilities to study even complex systems with a great level of detail. Due to the enormous progress in the field, quantitative systems biology studies of mixed-culture fermentations of plant-based milk alternatives could become a next level of research to better understand the underlying physiological, cellular and molecular processes. The system to be studied is admittedly complex, but seminal studies on similarly complex fermentation processes involving cocoa fermentation (Adler et al. 2013), oil-based riboflavin production (Schwechheimer et al. 2018), growth on substrate mixtures (Schilling et al. 2007) and under environmental changes (Hou et al. 2000; Kohajdová et al. 2006; Kohlstedt et al. 2014; Wittmann et al. 2007) are encouraging success stories, which demonstrate the power of systems biology to shed more light on this subject and provide valuable guidance for improvement. It can be expected that similar systems level studies, which unravel genomics, transcriptomics, proteomics, metabolomics, and fluxomics in multi-omics approaches, will significantly contribute to a better understanding of plant material fermentation and advance rational designs and improvements in this field. In addition, as the market becomes increasingly diverse, the fermentation of novel types of plant materials will become another important trend.
References
Adler P, Bolten CJ, Dohnt K, Hansen CE, Wittmann C (2013) Core fluxome and meta fluxome of lactic acid bacteria under cocoa pulp fermentation simulating conditions. Appl Environ Microbiol 79(18):5670–5681
Afaneh I, Abu-Alruz K, Quasem JM, Sundookah A, Abbadi J, Alloussi S, Ayyad Z (2011) Fundamental elements to produce sesame yoghurt from sesame milk. Am J Appl Sci 8(11):1086–1092
Anderson RL, Wolf WJ (1995) Compositional changes in trypsin inhibitors, phytic acid, saponins and isoflavones related to soybean processing. J Nutr 125(3):581S–588S
Angelov A, Gotcheva V, Hristozova T, Gargova S (2005) Application of pure and mixed probiotic lactic acid bacteria and yeast cultures for oat fermentation. J Sci Food Agric 85(12):2134–2141
Antony U, Chandra TS (1998) Antinutrient reduction and enhancement in protein, starch, and mineral availability in fermented flour of finger millet (Eleusine coracana). J Agric Food Chem 46(7):2578–2582
Asioli D, Aschemann-Witzel J, Caputo V, Vecchio R, Annunziata A, Næs T, Varela P (2017) Making sense of the “clean label” trends: a review of consumer food choice behavior and discussion of industry implications. Food Res Int 99:58–71
Bentley R, Meganathan R (1982) Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol Rev 46(3):241–280
Bernat N, Cháfer M, Chiralt A, González-Martínez C (2015) Development of a non-dairy probiotic fermented product based on almond milk and inulin. Food Sci Technol Int 21(6):440–453
Bloomberg Surveillance (2015) Got coke?: Coca-Cola’s big bet on premium milk. Bloomberg Surveillance. https://www.bloomberg.com/news/videos/2015-02-10/got-coke-coca-cola-s-big-bet-on-premium-milk. Accessed 10 Feb 2015
Briviba K, Gräf V, Walz E, Guamis B, Butz P (2016) Ultra high pressure homogenization of almond milk: physico-chemical and physiological effects. Food Chem 192:82–89
Callaway JC (2004) Hempseed as a nutritional resource: an overview. Euphytica 140(1-2):65–72
Champagne CP, Green-Johnson J, Raymond Y, Barrette J, Buckley N (2009) Selection of probiotic bacteria for the fermentation of a soy beverage in combination with Streptococcus thermophilus. Food Res Int 42(5-6):612–621
Champagne CP, Tompkins TA, Buckley ND, Green-Johnson JM (2010) Effect of fermentation by pure and mixed cultures of Streptococcus thermophilus and Lactobacillus helveticus on isoflavone and B-vitamin content of a fermented soy beverage. Food Microbiol 27(7):968–972
Chien HL, Huang HY, Chou CC (2006) Transformation of isoflavone phytoestrogens during the fermentation of soymilk with lactic acid bacteria and bifidobacteria. Food Microbiol 23(8):772–778
Chumchuere S, Robinson RK (1999) Selection of starter cultures for the fermentation of soya milk. Food Microbiol 16(2):129–137
Ciani M, Comitini F, Mannazzu I, Domizio P (2010) Controlled mixed culture fermentation: a new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res 10(2):123–133
Craig WJ (2010) Nutrition concerns and health effects of vegetarian diets. Nutr Clin Pract 25(6):613–620
Crittenden RG, Bennett LE (2005) Cow’s milk allergy: a complex disorder. J Am Coll Nutr 24:582S–591S
Dakwa S, Sakyi-Dawson E, Diako C, Annan NT, Amoa-Awua WK (2005) Effect of boiling and roasting on the fermentation of soybeans into dawadawa (soy-dawadawa). Int J Food Microbiol 104(1):69–82
DebMandal M, Mandal S (2011) Coconut (Cocos nucifera L.: Arecaceae): in health promotion and disease prevention. Asian Pac J Trop Med 4(3):241–247
Desai A, Small D, McGill AEJ, Shah NP (2002) Metabolism of raffinose and stachyose in reconstituted skim milk and of n-hexanal and pentanal in soymilk by bifidobacteria. Biosci Microflora 21(4):245–250
Drewnowski A, Gomez-Carneros C (2000) Bitter taste, phytonutrients, and the consumer: a review. Am J Clin Nutr 72(6):1424–1435
Duranti M, Consonni A, Magni C, Sessa F, Scarafoni A (2008) The major proteins of lupin seed: characterisation and molecular properties for use as functional and nutraceutical ingredients. Trends Food Sci Technol 19(12):624–633
Epstein SS (1990) Potential public health hazards of biosynthetic milk hormones. Int J Health Serv 20(1):73–84
Erbaş M, Certel M, Uslu MK (2005) Some chemical properties of white lupin seeds (Lupinus albus L.). Food Chem 89(3):341–345
Espirito-Santo AP, Mouquet-Rivier C, Humblot C, Cazevieille C, Icard-Vernière C, Soccol CR, Guyot JP (2014) Influence of cofermentation by amylolytic Lactobacillus strains and probiotic bacteria on the fermentation process, viscosity and microstructure of gruels made of rice, soy milk and passion fruit fiber. Food Res Int 57:104–113
Fernandez ML, Berry JW (1988) Nutritional evaluation of chickpea and germinated chickpea flours. Plant Foof Hum Nutr 38(2):127–134
Fujisawa K, Yokoyama A, Suzukamo G (1986) Lactic acid fermentation products of sunflower seed milk. US Patent 4563356, 01 Jul 1986
Gernand AD, Schulze KJ, Stewart CP, West KP Jr, Christian P (2016) Micronutrient deficiencies in pregnancy worldwide: health effects and prevention. Nat Rev Endocrinol 12(5):274–289
Ginsberg C, Ostrowski A (2007) The market for vegetarian foods. The Vegetarian Resource Group. http://www.vrg.org/nutshell/market.htm. Accessed 15 Aug 2007
Glanz K, Basil M, Maibach E, Goldberg J, Snyder D (1998) Why Americans eat what they do: taste, nutrition, cost, convenience, and weight control concerns as influences on food consumption. J Am Diet Assoc 98(10):1118–1126
Haug A, Høstmark AT, Harstad OM (2007) Bovine milk in human nutrition—a review. Lipids Health Dis 6(1):25
Horáčková Š, Mühlhansová A, Sluková M, Schulzová V, Plocková M (2015) Fermentation of soymilk by yoghurt and bifidobacteria strains. Czech J Food Sci 33(4)
Hou JW, Yu RC, Chou CC (2000) Changes in some components of soymilk during fermentation with bifidobacteria. Food Res Int 33(5):393–397
Hove EL (1974) Composition and protein quality of sweet lupin seed. J Sci Food Agric 25(7):851–859
Hugenschmidt S, Schwenninger SM, Lacroix C (2011) Concurrent high production of natural folate and vitamin B12 using a co-culture process with Lactobacillus plantarum SM39 and Propionibacterium freudenreichii DF13. Process Biochem 46(5):1063–1070
Hughes D (1995) Animal welfare: the consumer and the food industry. Br Food J 97(10):3–7
Ivanović D, Ballester D, Yanez E (1983) Formulation and nutritive value of 2 milk substitutes based on sweet lupine (Lupinus albus, var. multolupa). Arch Latinoam Nutr 33(3):620–632
Jeske S, Zannini E, Arendt EK (2017) Evaluation of physicochemical and glycaemic properties of commercial plant-based milk substitutes. Plant Foods Hum Nutr 72(1):26–33
Jeske S, Zannini E, Arendt EK (2018) Past, present and future: the strength of plant-based dairy substitutes based on gluten-free raw materials. Food Res Int 110:42–51
Jiang S, Cai W, Xu B (2013) Food quality improvement of soy milk made from short-time germinated soybeans. Foods 2(2):198–212
Juliano BO, Hicks PA (1996) Rice functional properties and rice food products. Food Rev Int 12(1):71–103
Kim WJ, Yoon SK, Lee CY (1986) Changes in oligosaccharides and sensory quality of soymilk during germination. Kor J Food Sci Technol 18(5):382–387
Kohajdová Z, Karovičová J, Greifová M (2006) Lactic acid fermentation of some vegetable juices. J Food Nutr Res 45(3):115–119
Kohlstedt M, Sappa PK, Meyer H, Maaß S, Zaprasis A, Hoffmann T, Becker J, Steil L, Hecker M, van Dijl JM (2014) Adaptation of Bacillus subtilis carbon core metabolism to simultaneous nutrient limitation and osmotic challenge: a multi-omics perspective. Environ Microbiol 16(6):1898–1917
Lai LR, Hsieh SC, Huang HY, Chou CC (2013) Effect of lactic fermentation on the total phenolic, saponin and phytic acid contents as well as anti-colon cancer cell proliferation activity of soymilk. J Biosci Bioeng 115(5):552–556
Lambo AM, Öste R, Nyman MEGL (2005) Dietary fibre in fermented oat and barley β-glucan rich concentrates. Food Chem 89(2):283–293
Lampart-Szczapa E, Korczak J, Nogala-Kalucka M, Zawirska-Wojtasiak R (2003) Antioxidant properties of lupin seed products. Food Chem 83(2):279–285
Lazaridou A, Biliaderis CG (2007) Molecular aspects of cereal β-glucan functionality: physical properties, technological applications and physiological effects. J Cereal Sci 46(2):101–118
Lebiedzińska A, Szefer P (2006) Vitamins B in grain and cereal-grain food, soy-products and seeds. Food Chem 95(1):116–122
LeBlanc JG, Laiño JE, Juarez del Valle M, Vannini V, van Sinderen D, Taranto MP, Font de Valdez G, Savoy de Giori G, Sesma F (2011) B-Group vitamin production by lactic acid bacteria–current knowledge and potential applications. J Appl Microbiol 111(6):1297–1309
LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M (2013) Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol 24(2):160–168
Lee C (2001) Changes in n-hexanal content of peanut milk fermented with lactic acid bacteria. Food Sci Biotechnol 10(4):387–390
Lee C, Beuchat LR (1991) Changes in chemical composition and sensory qualities of peanut milk fermented with lactic acid bacteria. Int J Food Microbiol 13(4):273–283
Leroy F, De Vuyst L (2004) Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci Technol 15(2):67–78
Li F, Cui R, Zheng L, Li C (2004) Study on the processing technology of pea milk with soya. Food Sci 8:211–214
Lindegren CC (1945) Yeast genetics: life cycles, cytology, hybridization, vitamin synthesis, and adaptive enzymes. Bacteriol Rev 9(3-4):111–170
Liu SQ, Tsao M (2009) Enhancement of survival of probiotic and non-probiotic lactic acid bacteria by yeasts in fermented milk under non-refrigerated conditions. Int J Food Microbiol 135(1):34–38
Liu JR, Chen MJ, Lin CW (2002) Characterization of polysaccharide and volatile compounds produced by kefir grains grown in soymilk. J Food Sci 67(1):104–108
Makinde FM, Akinoso R (2013) Nutrient composition and effect of processing treatments on anti nutritional factors of Nigerian sesame (Sesamum indicum Linn) cultivars. Int Food Res J 20(5):2293
Mäkinen OE, Wanhalinna V, Zannini E, Arendt EK (2016) Foods for special dietary needs: non-dairy plant-based milk substitutes and fermented dairy-type products. Crit Rev Food Sci Nutr 56(3):339–349
Matsuura M, Obata A, Fukushima D (1989) Objectionable flavor of soy milk developed during the soaking of soybeans and its control. J Food Sci 54(3):602–605
Millward DJ (1999) The nutritional value of plant-based diets in relation to human amino acid and protein requirements. Proc Nutr Soc 58(2):249–260
Mital BK, Steinkraus KH (1979) Fermentation of soy milk by lactic acid bacteria. A review. J Food Prot 42(11):895–899
Mital BK, Steinkraus KH, Naylor HB (1974) Growth of lactic acid bacteria in soy milks. J Food Sci 39(5):1018–1022
Mitchell CR, Mitchell PR, Nissenbaum R (1990) Nutritional rice milk product. US Patent 4894242, 16 Jan 1990
Moneret-Vautrin DA, Guérin L, Kanny G, Flabbee J, Frémont S, Morisset M (1999) Cross-allergenicity of peanut and lupine: the risk of lupine allergy in patients allergic to peanuts. J Allergy Clin Immunol 104(4):883–888
National Research Council (1992) Applications of biotechnology in traditional fermented foods. National Academies Press, Washington, D.C.
Noimark L, Cox HE (2008) Nutritional problems related to food allergy in childhood. Pediatr Allergy Immunol 19(2):188–195
Önning G, Åkesson B, Öste R, Lundquist I (1998) Effects of consumption of oat milk, soya milk, or cow’s milk on plasma lipids and antioxidative capacity in healthy subjects. Ann Nutr Metab 42(4):211–220
Onyesom I, Enaholo AT, Mordi J (2005) Effect of processing techniques on the contents of flatulence factors and emulsion properties of cowpea (Vigna unguiculata). J Appl Sci Environ Manag 9:2
Othman RA, Moghadasian MH, Jones PJH (2011) Cholesterol-lowering effects of oat β-glucan. Nutr Rev 69(6):299–309
Patisaul HB, Jefferson W (2010) The pros and cons of phytoestrogens. Front Neuroendocrinol 31(4):400–419
Paucar-Menacho LM, Berhow MA, Mandarino JMG, Chang YK, Mejia EG (2010) Effect of time and temperature on bioactive compounds in germinated Brazilian soybean cultivar BRS 258. Food Res Int 43(7):1856–1865
Pawlak R, Lester SE, Babatunde T (2014) The prevalence of cobalamin deficiency among vegetarians assessed by serum vitamin B12: a review of literature. Eur J Clin Nutr 68(5):541–548
Peyer LC, Zannini E, Arendt EK (2016) Lactic acid bacteria as sensory biomodulators for fermented cereal-based beverages. Trends Food Sci Technol 54:17–25
Pineli LDLDO, Botelho RBA, Zandonadi RP, Solorzano JL, de Oliveira GT, Reis CEG, Teixeira DDS (2015) Low glycemic index and increased protein content in a novel quinoa milk. LWT Food Sci Technol 63(2):1261–1267
Pyo YH, Lee TC, Lee YC (2005) Enrichment of bioactive isoflavones in soymilk fermented with β-glucosidase-producing lactic acid bacteria. Food Res Int 38(5):551–559
Ranhotra GS, Gelroth JA, Glaser BK, Lorenz KJ, Johnson DL (1993) Composition and protein nutritional quality of quinoa. Cereal Chem 70:303–303
Rao DR, Pulusani SR, Chawan CB (1988) Preparation of a yogurt-like product from cowpeas and mung beans. Int J Food Sci Technol 23(2):195–198
Rekha CR, Vijayalakshmi G (2010) Bioconversion of isoflavone glycosides to aglycones, mineral bioavailability and vitamin B complex in fermented soymilk by probiotic bacteria and yeast. J Appl Microbiol 109(4):1198–1208
Rotz CA, Montes F, Chianese DS (2010) The carbon footprint of dairy production systems through partial life cycle assessment. J Dairy Sci 93(3):1266–1282
Roy F, Boye JI, Simpson BK (2010) Bioactive proteins and peptides in pulse crops: pea, chickpea and lentil. Food Res Int 43(2):432–442
Rustom IYS, López-Leiva MH, Nair BM (1993) Extraction of peanut solids with water-effect of the process and enzymatic hydrolysis. LWT Food Sci Technol 26(1):72–75
Sandberg AS (2002) Bioavailability of minerals in legumes. Br J Nutr 88(S3):281–285
Sanni AI, Onilude AA, Adeleke EO (1999) Preparation and characteristics of lactic acid fermented cowpea milk. Z Lebensm Unters Forsch A 208(3):225–229
Santos CC, Libeck BS, Schwan RF (2014) Co-culture fermentation of peanut-soy milk for the development of a novel functional beverage. Int J Food Microbiol 186:32–41
Schilling O, Frick O, Herzberg C, Ehrenreich A, Heinzle E, Wittmann C, Stülke J (2007) Transcriptional and metabolic responses of Bacillus subtilis to the availability of organic acids: transcription regulation is important but not sufficient to account for metabolic adaptation. Appl Environ Microbiol 73(2):499–507
Schwechheimer SK, Becker J, Peyriga L, Portais JC, Wittmann C (2018) Metabolic flux analysis in Ashbya gossypii using 13C-labeled yeast extract: industrial riboflavin production under complex nutrient conditions. Microb Cell Factories 17(1):162
Seow CC, Gwee CN (1997) Coconut milk: chemistry and technology. Int J Food Sci Technol 32(3):189–201
Sethi S, Tyagi SK, Anurag RK (2016) Plant-based milk alternatives an emerging segment of functional beverages: a review. J Food Sci Technol 53(9):3408–3423
Sieuwerts S, de Bok FAM, Hugenholtz J, van Hylckama Vlieg JET (2008) Unraveling microbial interactions in food fermentations: from classical to genomics approaches. Appl Environ Microbiol 74(16):4997–5007
Škrbić B, Filipčev B (2008) Nutritional and sensory evaluation of wheat breads supplemented with oleic-rich sunflower seed. Food Chem 108(1):119–129
Smid EJ, Lacroix C (2013) Microbe–microbe interactions in mixed culture food fermentations. Curr Opin Biotechnol 24(2):148–154
Soetan KO, Oyewole OE (2009) The need for adequate processing to reduce the anti-nutritional factors in plants used as human foods and animal feeds: a review. Afr J Food Sci 3(9):223–232
Song YS, Frias J, Martinez-Villaluenga C, Vidal-Valdeverde C, Gonzalez de Mejia E (2008) Immunoreactivity reduction of soybean meal by fermentation, effect on amino acid composition and antigenicity of commercial soy products. Food Chem 108(2):571–581
Steinkraus KH (1997) Classification of fermented foods: worldwide review of household fermentation techniques. Food Control 8(5-6):311–317
Titiek FD, Umar S, Cahyanto MN, Takuya S, Endang SR, Kosuke N (2013) Effect of indigenous lactic acid bacteria fermentation on enrichment of isoflavone and antioxidant properties of kerandang (Canavalia virosa) extract. Int Food Res J 20(5):2945
Ulyatu F, Pudji H, Tyas U, Umar S (2015) The changes of sesaminol triglucoside and antioxidant properties during fermentation of sesame milk by Lactobacillus plantarum Dad 13. Int Food Res J 22(5):1945
Vacek J, Klejdus B, Lojková L, Kubán V (2008) Current trends in isolation, separation, determination and identification of isoflavones: a review. J Sep Sci 31(11):2054–2067
Vahanvaty US (2009) Hemp seed and hemp milk: the new super foods. Infant Child Adolesc Nutr 1(4):232–234
Vanga SK, Raghavan V (2018) How well do plant based alternatives fare nutritionally compared to cow’s milk? J Food Sci Technol 55(1):10–20
Vasquez-Orejarena E, Simons CT, Litchfield JH, Alvarez VB (2018) Functional properties of a high protein beverage stabilized with oat-β-glucan. J Food Sci 83(5):1360–1365
Verdeal K, Ryan DS (1979) Naturally-occurring estrogens in plant foodstuffs—a review. J Food Prot 42(7):577–583
Vidal-Valverde C, Frias J, Hernandez A, Martín-Alvarez PJ, Sierra I, Rodríguez C, Blazquez I, Vicente G (2003) Assessment of nutritional compounds and antinutritional factors in pea (Pisum sativum) seeds. J Sci Food Agric 83(4):298–306
Vilche C, Gely M, Santalla E (2003) Physical properties of quinoa seeds. Biosyst Eng 86(1):59–65
Villamide MJ, San Juan LD (1998) Effect of chemical composition of sunflower seed meal on its true metabolizable energy and amino acid digestibility. Poult Sci 77(12):1884–1892
Wang YC, Yu RC, Yang HY, Chou CC (2003) Sugar and acid contents in soymilk fermented with lactic acid bacteria alone or simultaneously with bifidobacteria. Food Microbiol 20(3):333–338
Wansink B, Sonka S, Goldsmith P, Chiriboga J, Eren N (2005) Increasing the acceptance of soy-based foods. J Int Food Agribus Mark 17(1):35–55
West LG, Greger JL, White A, Nonnamaker BJ (1978) In vitro studies on saponin-mineral complexation. J Food Sci 43(4):1342–1343
Wittmann C, Weber J, Betiku E, Krömer J, Böhm D, Rinas U (2007) Response of fluxome and metabolome to temperature-induced recombinant protein synthesis in Escherichia coli. J Biotechnol 132(4):375–384
Wood JA, Grusak MA (2007) Nutritional value of chickpea. In: Yadav S, Redden R, Chen W, Sharma B (eds) Chickpea breeding and management. CAB International, Wallingford, pp 101–142
Xu X, Wang HJ, Murphy PA, Cook L, Hendrich S (1994) Daidzein is a more bioavailable soymilk isoflavone than is genistein in adult women. J Nutr 124(6):825–832
Yamanaka Y, Okumura S, Mitsugi K, Hasegawa Y (1970) Method of preparing a sour milk beverage. US Patent 3535117, 20 Oct 1970
Yuan S, Chang SKC, Liu Z, Xu B (2008) Elimination of trypsin inhibitor activity and beany flavor in soy milk by consecutive blanching and ultrahigh-temperature (UHT) processing. J Agric Food Chem 56(17):7957–7963
Zamora AF, Fields ML (1979) Nutritive quality of fermented cowpeas (Vigna sinensis) and chickpeas (Cicer arietinum). J Food Sci 44(1):234–236
Zhang H, Önning G, Triantafyllou AÖ, Öste R (2007) Nutritional properties of oat-based beverages as affected by processing and storage. J Sci Food Agric 87(12):2294–2301
Zhao D, Shah NP (2014) Changes in antioxidant capacity, isoflavone profile, phenolic and vitamin contents in soymilk during extended fermentation. LWT Food Sci Technol 58(2):454–462
Funding
This study was funded by Nestec Ltd. (Vevey, Switzerland), which has been merged with Société des Produits Nestlé SA on 1st of June 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Christoph J. Bolten and Jeroen Muller are employed by Nestlé Research, Switzerland, which is part of Société des Produits Nestlé SA, a wholly owned subsidiary of Nestlé SA. Muzi Tangyu and Christoph Wittmann declare that they have no conflict of interest.
Ethical approval
This mini-review does not contain any studies with human participants or animals performed by any of the authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tangyu, M., Muller, J., Bolten, C.J. et al. Fermentation of plant-based milk alternatives for improved flavour and nutritional value. Appl Microbiol Biotechnol 103, 9263–9275 (2019). https://doi.org/10.1007/s00253-019-10175-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10175-9