Abstract
The natural interspecies Saccharomyces cerevisiae × Saccharomyces eubayanus hybrid yeast is responsible for global lager beer production and is one of the most important industrial microorganisms. Its success in the lager brewing environment is due to a combination of traits not commonly found in pure yeast species, principally low-temperature tolerance, and maltotriose utilization. Parental transgression is typical of hybrid organisms and has been exploited previously for, e.g., the production of wine yeast with beneficial properties. The parental strain S. eubayanus has only been discovered recently and newly created lager yeast strains have not yet been applied industrially. A number of reports attest to the feasibility of this approach and artificially created hybrids are likely to have a significant impact on the future of lager brewing. De novo S. cerevisiae × S. eubayanus hybrids outperform their parent strains in a number of respects, including, but not restricted to, fermentation rate, sugar utilization, stress tolerance, and aroma formation. Hybrid genome function and stability, as well as different techniques for generating hybrids and their relative merits are discussed. Hybridization not only offers the possibility of generating novel non-GM brewing yeast strains with unique properties, but is expected to aid in unraveling the complex evolutionary history of industrial lager yeast.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Beer and other fermented beverages have been produced for thousands of years and have played an important part in most human societies (Hornsey 2003, 2012). Yeast (primarily of the Saccharomyces genus) play a vital role in beer production and quality; during fermentation, they not only convert wort carbohydrates into ethanol and CO2, but also synthesize various key flavor compounds. Traditionally, brewer’s yeasts have been divided into top- and bottom-fermenting strains depending on their fermentation behavior, but modern molecular techniques have revealed high diversity between yeast strains used for brewing (Gallone et al. 2016; Legras et al. 2007; Liti et al. 2005; Steensels et al. 2014). Moreover, many of the yeast strains that brewers have used for centuries have now been shown to be interspecific hybrids. In particular, lager yeast or Saccharomyces pastorianus, the workhorse of the modern brewing industry, is known to be an interspecific hybrid between Saccharomyces cerevisiae and the cold-tolerant Saccharomyces eubayanus (de Barros Lopes et al. 2002; Dunn and Sherlock 2008; Libkind et al. 2011; Liti et al. 2005; Nilsson-Tillgren et al. 1981; Tamai et al. 1998). In addition, natural hybrids between S. cerevisiae and Saccharomyces kudriavzevii have been isolated from Belgian Trappist beers (González et al. 2008), while natural hybrids between S. cerevisiae and Saccharomyces uvarum are used frequently in winemaking (Le Jeune et al. 2007). The hybrid state appears to confer a competitive advantage in the fermentation environment. In the case of the lager yeast, success has been due to a fortunate combination of phenotypes. Low-temperature fermentation was enabled by the inheritance of cryotolerance from S. eubayanus, while efficient maltotriose utilization and other beneficial fermentation properties were inherited from S. cerevisiae (Hebly et al. 2015; Krogerus et al. 2015). Despite the industrial importance of lager yeasts, much of their natural history remains obscure.
The hybrid nature of S. pastorianus had been suspected for some time. Early research, particularly from the Carlsberg Laboratory in Copenhagen, showed that the lager yeast genome included genetic material derived from S. cerevisiae and a non-S. cerevisiae yeast (Nilsson-Tillgren et al. 1981; Pedersen 1985; Hansen et al. 1994). As early as 1944, Øjvinde Winge had described the poor sporulation ability of lager yeast; an indication that they did not represent a pure species (Winge 1944). The exact composition of the hybrid in terms of parentage and ploidy did not begin to become properly resolved until the application of genomic studies to lager yeast. In key papers, Liti et al. (2005) and Dunn and Sherlock (2008) showed that the lager yeast could be divided into two genetically distinct groups and that these corresponded exactly with the traditional Saaz and Frohberg designations used by brewers (Glendinning 1899). Both groups contained S. cerevisiae and Saccharomyces bayanus-like DNA, though the Saaz or group 1 yeast contained proportionally more S. bayanus-type DNA. The Frohberg (group II) yeast contained relatively more DNA, appearing to be triploid rather than diploid based on the CGH-array analysis. Whole-genome analysis later revealed the Saaz and Frohberg groups to be triploid and tetraploid, respectively (Nakao et al. 2009; Walther et al. 2014). A further breakthrough came in 2011 with the first report of S. eubayanus, which had been isolated from Patagonia, where it was found associated with Nothofagus (Libkind et al. 2011). Genetic analysis revealed that the non-S. cerevisiae moiety of the lager yeast genome was almost certainly derived from S. eubayanus.
It has generally been assumed that the initial hybridization between the parental strains occurred when S. eubayanus contaminated a traditional brewery fermentation (Gibson and Liti 2015). This scenario is supported by the fact the S. cerevisiae component of the lager yeast genome seems to more closely resemble ale strains of S. cerevisiae than wild strains (Dunn and Sherlock 2008; Monerawela et al. 2015), though definitive proof is still lacking and it may be too early to discount the possibility of the progenitor being, e.g., a wild S. cerevisiae × S. eubayanus hybrid. A further assumption is that this event occurred in Central Europe in approximately the sixteenth century, based on the advent of lager brewing in this area and at this time. It is tempting to speculate that the 1533 prohibition of brewing in Bavaria during the summer months (Dornbusch, 1997) provided the conditions necessary for the competitive success of the cryotolerant S. pastorianus hybrid relative to traditional ale strains. It is, however, likely that such assumptions will be reassessed or possibly discarded as more information on the lager yeast genome and the ecology of the parental species becomes available.
Since the original isolation of S. eubayanus in South America, there have been a number of isolations elsewhere, including from North America (Peris et al. 2014, 2016), East Asia (Bing et al. 2014), and New Zealand (Gayevskiy and Goddard 2015) but, interestingly, not as yet from Europe. Recent sequence analysis suggests that there may be a Northern Hemisphere, or “Holarctic,” group of related strains with a wide geographical distribution, comprised (so far) of certain strains found in Tibet and in North America (Peris et al. 2016). Furthermore, it appears that it is a combination of the standing variation found among the Tibetan and North American strains that most closely matches the S. eubayanus parent of lager yeast (Peris et al. 2016). It therefore cannot be concluded with any certainty that the parental S. eubayanus strain came directly from Asia as suggested by the “Silk Road” hypothesis (Bing et al. 2014). Rather, an undiscovered population is most likely resident in Europe and individuals from this population were probably involved in the original hybridization event (or events) that gave rise to the lager yeast. A comparable situation has been observed with S. kudriavzevii which exists in Europe but was only found after suitable methodology was developed for its isolation (Sampaio and Gonçalves 2008; Lopes et al. 2010). Until this time, the species had only been found in hybrid form with S. cerevisiae in European vineyards and was only known to occur in its pure form in Asia (Naumov et al. 2000). Our understanding of the natural ecology of wild Saccharomyces species is severely limited (Goddard and Greig 2015) and it is probable that S. eubayanus (and other Saccharomyces species) will eventually be uncovered in Europe from an unexplored ecological niche.
Interspecific hybrids are not only used in brewing, but are commonly exploited in agriculture in order to significantly improve animal and crop yields (Chen 2013; Fu et al. 2015; Schnable and Springer 2013). This is because hybrid species often exhibit superior phenotypic qualities relative to parent strains, i.e., heterosis or hybrid vigor, and are chosen for their improved growth rates and crop yields. Phenotype amplification and heterosis have also been observed in studies on de novo yeast hybrids, which have exhibited a range of improved traits including faster fermentation rates, more complete sugar use, greater stress tolerance, and increases in aroma compound production (Bellon et al. 2011, 2013, 2015; Dunn et al. 2013; Gamero et al. 2013; Hebly et al. 2015; Krogerus et al. 2015, 2016; Mertens et al. 2015; Piotrowski et al. 2012; Plech et al. 2014; Snoek et al. 2015; Steensels et al. 2014). Interspecific hybridization can be seen as a powerful strain development tool for brewing yeast, one which enables the combination and enhancement of phenotypic features from different parent strains. Moreover, these new hybrid strains can be generated without the use of targeted genetic modification, the use of which in the brewing industry still remains limited as a result of regulations and public opinion (Twardowski and Malyska 2015). In addition to their immediate industrial applications, these new yeast hybrids may also help to elucidate the evolutionary history of industrial hybrid yeast strains, which still remains a subject of debate (Baker et al. 2015; Okuno et al. 2016; Peris et al. 2016).
This review will discuss the use of hybridization as a strain development tool for brewery applications, with particular focus on the recent research that has been carried out on de novo lager yeast. First, studies on the creation and use of hybrid yeast in brewery environments will be summarized. Then, specific industry-relevant phenotypes will be described individually. In addition, hybrid genome regulation and stability will also be discussed briefly. Finally, methods for generating interspecific yeast hybrids will be summarized and their relative merits discussed. Discussion on natural lager yeast hybrids will be kept to a minimum, as this topic has recently been reviewed elsewhere (Gibson and Liti 2015; Wendland 2014).
Artificial hybrids
The generation of yeast hybrids, mainly for ale brewing purposes, has been carried out for decades already (Hammond and Eckersley 1984; Johnston 1965; Russell et al. 1983; Spencer and Spencer 1977). Early work involved the breeding of S. cerevisiae ale and laboratory strains in attempts to create intraspecific hybrids with improved fermentation rates and attenuation (Johnston 1965; Spencer and Spencer 1977). However, applying classic yeast breeding to brewing yeast is challenging, as industrial brewing strains often suffer from poor sporulation efficiencies and viabilities, presumably as a result of aneuploidy (Bilinski et al. 1986; Codón et al. 1995). Low fertility can be overcome through the use of rare mating or protoplast fusion, neither of which require the use of spores or haploid cells. These techniques have been used, e.g., to introduce dextrin fermentation from S. cerevisiae (syn. S. cerevisiae var. diastaticus) to both ale and lager yeast (Choi et al. 2002; Russell et al. 1983; Tubb et al. 1981), to improve the flocculation of industrial brewing strains through electrofusion (Urano et al. 1993), and to improve the ester formation and fermentation rate of ale yeast through fusion with a sake yeast (Mukai et al. 2001). More recently, selection and breeding of intraspecific hybrids with superior aroma compound production from pools of hundreds of parent strains has been accomplished using modern robot-assisted high-throughput techniques (Steensels et al. 2014).
Breeding of lager yeast has also been attempted previously, but is relatively difficult due to their aneuploidy and hybrid nature (Dunn and Sherlock 2008; Greig et al. 2002; Pfliegler et al. 2012). These result in low sporulation efficiencies, spore viabilities, and mating frequency, as was revealed by early work on Saaz-type S. pastorianus at Carlsberg (Gjermansen and Sigsgaard 1981). More recently, spore clones from presumably the same Saaz-type S. pastorianus strain were crossed with an S. cerevisiae ale strain to yield hybrids with improved growth at higher temperatures and resistance to high ethanol concentrations (Garcia Sanchez et al. 2012). Breeding with Frohberg-type S. pastorianus strains is also limited by low sporulation frequencies (Ogata et al. 2011). Again, as was previously discussed, these limitations can be overcome through the use of rare mating or protoplast fusion, which have also been successfully applied to lager yeast (Janderová et al. 1990; Russell et al. 1983; Sato et al. 2002).
Another factor that limited the breeding of lager yeast was the absence of the non-S. cerevisiae parent. However, the recent discovery of S. eubayanus (Libkind et al. 2011) has permitted the creation of novel artificial lager yeast hybrids (Alexander et al. 2016; Hebly et al. 2015; Krogerus et al. 2015, 2016; Mertens et al. 2015). These hybrids possess great potential value for the brewing industry, as it has been shown that they may ferment faster, possess a broader temperature tolerance range, and produce more diverse aroma compounds than their parent strains. Recent studies on such hybrids have been restricted mainly to hybridization with the S. eubayanus type strain (CBS 12357), which alone has been shown to perform poorly in wort fermentations relative to lager yeast strains (Gibson et al. 2013). Nevertheless, it does possess many traits advantageous for lager brewing, such as low-temperature growth (down to 4 °C), efficient maltose use, and production of desirable aroma compounds, which can be inherited by the hybrids (Gibson et al. 2013; Hebly et al. 2015, Krogerus et al. 2015, 2016; Mertens et al. 2015). It is expected that the diversity of new lager yeast strains will increase in the near future as new isolates of S. eubayanus become available for mating.
Aside from lager yeast hybrids, the use of de novo interspecific hybrids created from other species in the Saccharomyces genus (i.e., Saccharomyces arboricola, Saccharomyces kudriavzevii, Saccharomyces mikatae, Saccharomyces paradoxus, or S. uvarum) for brewing purposes has not been explored. However, recent studies on the use of de novo S. cerevisiae interspecific hybrids with S. kudriavzevii (Bellon et al. 2011; Lopandic et al. 2016), S. mikatae (Bellon et al. 2013), S. paradoxus (Bellon et al. 2011), and S. uvarum (Bellon et al. 2015; Lopandic et al. 2016) for wine making have revealed the potential for increasing aromatic diversity and fermentation performance. As many of these “alternative” Saccharomyces species are also cold-tolerant, e.g., S. kudriavzevii and S. uvarum (Gonçalves et al. 2011; López-Malo et al. 2013; Paget et al. 2014), they represent feasible alternatives to S. eubayanus in interspecific hybrids for lager brewing purposes and may compensate for the current paucity of S. eubayanus isolates. Another group of hybrids, that currently remains poorly explored in relation to brewing applications, is the intergeneric hybrid group. Such hybrids have been successfully constructed through protoplast fusion (Lucca et al. 2002; Spencer et al. 1983). Species belonging formerly to the Saccharomyces sensu lato group may be of particular interest in this regard. Interest in the potential of non-Saccharomyces yeasts in brewing has increased in recent years (Basso et al. 2016; Canonico et al. 2016; Michel et al. 2016). However, it remains to be seen if such strain development approaches will find acceptance for industrial-scale brewing.
In brief, de novo yeast hybrids have been used successfully to improve beer fermentation in a number of respects, including fermentation rate, aroma formation, and stress tolerance. The following section will discuss in more detail how various phenotypes important for beer fermentation can be affected in these yeast hybrids. A list of recent and relevant studies investigating the use of de novo yeast hybrids for beer fermentation has been compiled in Table 1.
Hybrid phenotypes
Aroma production
During wort fermentation, yeast produce a range of metabolites which contribute to beer aroma. The main groups of yeast-derived aroma-active compounds in beer are higher alcohols, esters, sulfur compounds, volatile phenols, vicinal diketones, and aldehydes (for recent reviews, see Krogerus and Gibson 2013; Landaud et al. 2008; Pires et al. 2014; Vanderhaegen et al. 2006). However, not all yeast-derived aroma compounds are desirable. Thus, the target of many strain development strategies is to increase the production of certain aroma compounds, such as esters, while decreasing the production of off-aromas, such as vicinal diketones and sulfur compounds. Hybridization offers a valuable alternative, as diverse aroma phenotypes can be combined, and increased aroma formation can be achieved through best-parent heterosis.
Studies on yeast hybrids in beverage fermentation have revealed the possibility of either increasing aroma production or achieving mid-parent values in hybrids (Bellon et al. 2011, 2013; da Silva et al. 2015; Gamero et al. 2013; Krogerus et al. 2015, 2016; Mertens et al. 2015; Mukai et al. 2001; Steensels et al. 2014). Early work by Mukai et al. (2001) showed that the concentrations of 2-methylpropyl acetate (fruit aroma) and ethyl hexanoate (apple/aniseed aroma) in beer could be increased by using an ale × sake intraspecific hybrid compared to the ale parent strain. More recently, a large-scale breeding study by Steensels et al. (2014) revealed that a 45 % increase in 3-methylbutyl acetate (banana aroma) formation could be achieved in intraspecific hybrids. Outbred hybrids, i.e., those formed by hydridization between spores (segregants) from two different parent strains, in particular tended to show a greater increase in ester production compared to inbred hybrids, i.e., those formed by hydridization between spores (segregants) derived from a single parent strain. It has been shown that heterosis in regard to the growth rates of yeast hybrids formed from domesticated parent strains is positively correlated with sequence divergence (Plech et al. 2014; Shapira et al. 2014). However, Steensels et al. (2014) did not see an increase in 3-methylbutyl acetate formation as the genetic distance of the parent strains was increased. Nevertheless, it is possible that this effect is more pronounced in interspecific hybrids compared to intraspecific hybrids as suggested, e.g., in the study by da Silva et al. (2015) with regard to ethyl ester formation.
Probably due to the close relatedness of natural lager yeast hybrids (Dunn and Sherlock 2008; Okuno et al. 2016), a limited aroma spectrum exists within this group (Gibson et al. 2013; Mertens et al. 2015). It was recently shown that this diversity can be improved by generating new interspecies lager hybrids (Mertens et al. 2015). Results revealed that the aroma profiles of these strains ranged from worst- to best-parent levels, with several of the hybrids producing higher concentrations of aroma compounds than either of their parents (especially 3-methylbutyl and 2-methylpropyl acetate). A similar result was obtained in other work (Krogerus et al. 2015), where de novo lager hybrids produced beers with higher overall concentrations of esters compared to the parent strains. Certain esters, such as ethyl hexanoate, were formed at higher concentrations than either parent strain, and above the flavor threshold. In a follow-up study, during which hybrids with different ploidy from the same parent strains were compared, it was further shown that the aroma profile of hybrids can be controlled based on the relative contribution of parental DNA (Krogerus et al. 2016). The highest concentrations of ethyl and acetate esters were produced by the tetraploid hybrid (Figure 1), while the triploid hybrid (containing proportionally more of the S. cerevisiae parent genome) formed lower amounts of acetate esters which were associated with the S. eubayanus parent strain. Transcriptional analysis and copy number estimation of several key genes related to the synthesis of these ethyl and acetate esters suggested that these observed differences can be partly attributed to higher gene copy numbers and transcription levels at higher ploidy. It was recently revealed that orthologous alcohol acetyltransferases (i.e., Atf1 and Atf2) derived from various Saccharomyces species show differences in their functional properties (Stribny et al. 2016), which may also contribute to the more diverse aroma formation that has been observed in de novo lager hybrids.
The a alcohol content, b percentage of maltotriose consumed, c final 3-methylbutyl acetate concentration (mg/L), and d final ethyl hexanoate concentration (mg/L) of a 15 °P all-malt wort fermented at 15 °C with an S. cerevisiae A81062 ale strain, the S. eubayanus C12902 type strain, and an allotetraploid interspecific lager hybrid (hybrid C4) between the two. Values are means from two independent fermentations and error bars where visible represent the standard deviation. A solution of X °P has the same density as an aqueous sucrose solution containing X g of sucrose in 100 g of solution. The figure was recreated using data from Krogerus et al. (2016)
While hybridization can be used to modify the production of desirable aroma compounds, one must be aware of the inherent risk of simultaneously increasing the formation of undesirable off-flavors. The formation of ethyl acetate, which is unpleasant at high concentrations, is positively correlated with 3-methylbutyl acetate formation, and thus hybrids with increased levels of the latter tend to show higher levels of the former (Steensels et al. 2014). The formation of the unwanted vicinal diketone diacetyl may also increase in hybrid strains compared to their parents (Krogerus et al. 2016). However, hybridization can also be used to decrease or completely remove unwanted aroma compounds associated with one of the parent strains. For instance, Tubb et al. (1981) removed the ability to produce 4-vinylguaiacol (phenolic off-flavor) from a dextrin-fermenting S. cerevisiae strain by first mating it with an ale strain and then screening meiotic segregants of this hybrid for low 4-vinyl guaiacol production. The phenolic off-flavor phenotype has been attributed to functional PAD1 and FDC1 genes (Mukai et al. 2014), and recent whole-genome sequencing of industrial brewing strains has revealed that strains lacking this phenotype contain loss-of-function mutations in either of these genes (Gallone et al. 2016; Gonçalves et al. 2016). Gallone et al. (2016) also demonstrated that hybrid strains lacking the ability to produce 4-vinyl guaiacol can be constructed if both of the parent strains contain such loss-of-function mutations in either PAD1 or FDC1. Similarly, Bizaj et al. (2012) were able to decrease the formation of H2S (rotten egg aroma) in hybrid wine strains by mating them with a low H2S-producing S. cerevisiae wine strain. We have also observed that the S. eubayanus type strain, which has been used to create the majority of de novo lager hybrids, can produce sulfuric off-aromas (e.g., ethanethiol and ethyl thioacetate) during wort and must fermentations (our unpublished data). These traits may also transfer to any hybrids formed from this strain. However, such hybrids tend to produce decreased mid-parent concentrations of these unwanted sulfuric compounds (our unpublished data). In conclusion, hybridization can be used as a means to increase the production of desirable aroma compounds and decrease the production of unpleasant volatiles relative to the parent strains.
Temperature tolerance
The ability to tolerate low temperatures is one of the defining characteristics of lager yeast and permits the low-temperature fermentation necessary for production of lager beer. This style is characterized by a clean aroma relative to the more intense fruit and floral notes characteristic of ales, a difference that is mostly due to the different fermentation temperatures employed. Low-temperature lager fermentations require the yeast to be able to survive and stay metabolically active in the cold (Gibson and Liti 2015). It is known that the cold tolerance of S. pastorianus is a result of its hybrid nature. However, the mechanisms by which this yeast and its cold-tolerant parent S. eubayanus cope with low temperatures are not known. As S. eubayanus has only recently been discovered, we have a limited understanding of the metabolic processes responsible for its superior cryotolerance. Gibson et al. (2013) showed with lager yeast that the more dominant the S. eubayanus genome portion is, the more cold-tolerant the strain is. For instance, Saaz-type strains are better adapted to cold than Frohberg-type. The presence of α-glucoside transporters that function better at lower temperatures, such as Mtt1, could play a role in the yeast performance at these temperatures (Vidgren et al. 2010 2014; Magalhães et al. 2016). Artificial interspecific hybrids of S. cerevisiae and S. eubayanus strains clearly have the ability to efficiently ferment wort at temperatures as low as 12 °C (Krogerus et al. 2015; Hebly et al. 2015; Mertens et al. 2015). How the hybrid genomes cooperate to produce this kind of phenotype is not yet clear.
In addition to S. eubayanus, other members of the Saccharomyces genus, such as S. kudriavzevii and S. uvarum, are also adept at growing and fermenting at low temperatures (González et al. 2006; Masneuf-Pomarède et al. 2010). These species are usually associated with wine and cider fermentation (González et al. 2006; Naumov et al. 2001). In the case of S. kudriavzevii, only interspecific hybrids have been found in fermentation conditions (Sampaio and Gonçalves 2008; Lopes et al. 2010). Cold-tolerant S. uvarum strains show higher ethanol sensitivity in wine fermentations at warmer temperatures (25 °C) than they do at low temperatures (13 °C), possibly due to a different fatty acid composition of the cell (Kishimoto et al. 1994; Masneuf-Pomarède et al. 2010). We have observed a similar behavior from the S. eubayanus-type strain, with it being sensitive to ethanol at warm temperatures but not affected in the cold (unpublished data). The response of these species to the combined effect of temperature and ethanol in the cell membrane deserves further investigation.
Low temperature is known to affect the efficiency of protein translation, fluidity of the membrane, lipid composition, protein folding, stability of messenger RNA (mRNA) structures and enzymatic activities (Aguilera et al. 2007; Sahara et al. 2002; Schade et al. 2004; Tai et al. 2007). Salvadó et al. (2011) showed, prior to the discovery of S. eubayanus, that S. kudriavzevii had the lowest optimal growth temperature of all the Saccharomyces species. Gonçalves et al. (2011) compared the rate of adaptation between S. cerevisiae and S. uvarum and found that groups of genes associated with cell wall mannoproteins, ribosomal stalk, translation elongation factors, and glycolysis have undergone “accelerated” evolution. Paget et al. (2014) identified genes associated with glycerol and acetaldehyde metabolism as being responsible for the cryotolerance of S. kudriavzevii and were able to replicate this effect by overexpressing the genes in S. cerevisiae. García-Ríos et al. (2016) further observed that S. kudriavzevii is better adapted to grow at low temperatures due to more efficient protein translation. This is true also for cold-adapted S. cerevisiae strains (Salvadó et al. 2016). None of these studies has however included S. eubayanus, and although similar mechanisms may be involved in the cold tolerance of this species, different species are known to react differently to variations in temperature. For example, S. uvarum improves respiration rates at low temperatures while S. kudriavzevii has superior ethanol production under similar conditions (Gonçalves et al. 2011).
Lager yeast hybrids clearly benefit from the cryotolerance conferred by S. eubayanus. The exceptional cold tolerance of this species is illustrated by the fact that even a cold-tolerant species like S. uvarum can benefit from the relationship. Almeida et al. (2014) have shown that domesticated strains of S. uvarum, i.e., those used in low-temperature cider and wine fermentations, contain introgressed DNA from S. eubayanus. Such introgressions are typically absent in wild strains of S. uvarum and the genetic contribution from S. eubayanus appears to be the main differentiating factor between wild and domesticated strains of the species. The origin of this genetic material has yet to be determined, i.e., directly from a natural population of S. eubayanus or indirectly via interaction with an existing S. eubayanus hybrid.
Sugar utilization
Maltose and maltotriose are the main fermentable sugars of wort. The parent strains of lager hybrids have different sugar utilization characteristics. Brewing strains of S. cerevisiae are usually able to utilize both maltose and maltotriose efficiently, whereas S. eubayanus appears able to utilize only maltose (Gallone et al. 2016; Gibson et al. 2013; Hebly et al. 2015). This seems to be due to a lack of maltotriose transporters (Hebly et al. 2015; Baker et al. 2015). However, so far, only one Patagonian isolate, the S. eubayanus type strain CBS12357, has been characterized in terms of maltotriose utilization (Gibson et al. 2013; Hebly et al. 2015) and newly found strains, e.g., Northern Hemisphere isolates from China and North America (Bing et al. 2014; Peris et al. 2016) and the New Zealand isolate (Gayevskiy and Goddard 2016) remain to be characterized. The two subgroups of the lager yeasts, Saaz and Frohberg, also differ in their sugar utilization characteristics. Frohberg strains can utilize both maltose and maltotriose, whereas the more S. eubayanus-like Saaz strains are in general unable to ferment maltotriose, resulting in lower growth and fermentation rates (Gibson et al. 2013; Magalhães et al. 2016). This might be because Saaz strains lost significant portions of their S. cerevisiae genome after hybridization (Dunn and Sherlock 2008; Walther et al. 2014) and possibly lost genes needed for maltotriose utilization during this reorganization of the genome. On the other hand, some authors suggest that Saaz and Frohberg lineages were created by two distinct hybridization events between different ale strains (Dunn and Sherlock 2008; Baker et al. 2015; Monerawela et al. 2015) or even between different S. eubayanus strains (Baker et al. 2015) possibly possessing different maltotriose utilization genes, which might explain differences in maltotriose utilization seen between the groups.
In general, at the low temperatures used for lager brewing (8–15 °C), de novo lager hybrids outperform the parental strains in terms of maltose and, especially, maltotriose utilization rates (Krogerus et al. 2015, 2016; Mertens et al. 2015). De novo interspecific hybrids have even displayed similar fermentation efficiencies to S. pastorianus strains currently used for commercial beer production (Krogerus et al. 2015; Mertens et al. 2015). The majority of the 31 interspecific lager hybrids created by Mertens et al. (2015) outperformed the parental strains in regards to ethanol production during fermentations at 16 °C. Three of the hybrids (all from different S. cerevisiae parents crossed with the Y567 Patagonian isolate of S. eubayanus) showed an ethanol production capacity similar or higher to the best reference S. pastorianus strains. The difference in ethanol production between strains was shown to be largely due to the ability to efficiently ferment maltotriose present in the wort. Strains producing less than 5 % alcohol by volume only fermented 50–60 % of the available maltotriose, whereas strains producing more than 5 % ethanol fermented up to 70 % of the maltotriose.
In the studies of Krogerus et al. (2015, 2016), lager hybrids resulting from a cross between an ale strain and the S. eubayanus type strain were also observed to ferment more efficiently than the parental strains (Figure 1). All hybrids had inherited the maltotriose uptake ability of the S. cerevisiae parent as well as the cold tolerance of S. eubayanus, enabling successful growth and fermentation at low temperatures (12 and 15 °C). The sugar profiles of the original wort and the beers produced revealed that the greater sugar uptake relative to the S. eubayanus parent was a result of efficient maltotriose utilization. The S. cerevisiae parent had only limited ability to utilize wort sugars at lower temperatures and there was a significant amount of residual maltose in the resulting beer. Krogerus et al. (2016) also revealed that the ploidy of de novo lager hybrids influences fermentation performance, as the hybrid strains with higher DNA content (i.e., the allotetraploid hybrid followed by the allotriploid hybrid) were clearly superior to lower ploidy hybrids in the fermentation of wort at 15 °C. There was a clear link between fermentation performance of hybrids and different sugar consumption abilities during fermentation, as strains fermenting fastest also consumed maltose and maltotriose fastest. As it is the uptake of maltose and maltotriose that tends to limit fermentation capacity during brewing (Alves et al. 2007; Rautio and Londesborough 2003), higher ploidy of hybrids would provide for a greater number of maltose/maltotriose transporter genes in hybrid genomes, which could account for improved uptake of these sugars. The interspecific hybrid between S. eubayanus type strain and the S. cerevisiae IMK439 laboratory strain studied by Hebly et al. (2015) also inherited the cryotolerance of S. eubayanus and maltotriose utilization ability of the S. cerevisiae parent. Additionally, it was able to grow more rapidly on maltose at 20 °C, resulting in a fermentation time that was 10 h shorter compared to S. cerevisiae parent.
So far, only the Patagonian S. eubayanus isolates and, in particular, the type strain CBS12357, have been used as the non-S. cerevisiae parent in S. cerevisiae × S. eubayanus crosses (Hebly et al. 2015; Krogerus et al. 2015, 2016; Mertens et al. 2015). As S. eubayanus apparently cannot use maltotriose, industrial lager strains seem to have inherited this trait from the original S. cerevisiae parent. However, as discussed in the “Introduction” section, results from whole-genome sequencing of recently discovered S. eubayanus strains have shown that isolates from the Northern hemisphere, North America and Tibet in particular, seem to be the closest relatives to the domesticated S. eubayanus half of the lager hybrid (Bing et al. 2014; Peris et al. 2016). This raises the question of whether there may exist S. eubayanus lineages capable of maltotriose uptake. Interestingly, analysis of the genome sequence of the Tibetan S. eubayanus isolate (Bing et al. 2014) identified ORFs that exhibited better similarity with AGT1 than with MAL31 (Hebly et al. 2015) suggesting that the Tibetan S. eubayanus might actually possess a maltotriose transporter gene (AGT1) found to be missing from the complete genome assembly of the Patagonian S. eubayanus strain (Baker et al. 2015). However, the ability of newly isolated North American strains and of the Tibetan strain to grow on maltotriose remains to be assessed (Bing et al. 2014; Peris et al. 2016).
Aside from fermentable sugars such as maltose and maltotriose, wort contains a large share of non-fermentable carbohydrates, the most abundant of which is dextrin. Its utilization during fermentation would result in higher ethanol yields and lower-carbohydrate beer. Some strains of S. cerevisiae (syn. S. cerevisiae var. diastaticus) have been shown to ferment dextrin, and this ability has been transferred to both ale and lager yeast through hybridization (Choi et al. 2002; Russell et al. 1983; Tubb et al. 1981). These hybrids showed higher fermentation degrees and ethanol yields than the brewing yeast parents.
Hybrid genome function and stability
It might be expected that in newly formed interspecific hybrids, there is a certain level of functional disorder due to the clash of different regulatory networks, and consequently, that this disorder has an influence on the evolution of the genome. Relatively little is known of the transregulation of gene activity in de novo hybrids. Bolat et al. (2013) have shown that removal of the S. eubayanus allele of the regulator ARO80 from a production strain of S. pastorianus did not significantly affect expression of the S. eubayanus form of the target gene ARO10. Results suggested that the S. cerevisiae regulator could compensate entirely for the loss and, that at least in natural S. pastorianus strains, cooperative mechanisms exist between subgenomes. Proteome and transcriptome studies have, however, shown that significant differences in subgenome activity can occur in lager yeast (Caesar et al. 2007; Horinouchi et al. 2010; Minato et al. 2009; Yoshida et al. 2007), suggesting that gene regulation in interspecies hybrids is not seamlessly integrated across subgenomes. Gibson et al. (2015) showed that differences in expression of the two different alleles of the regulatory gene ILV6 in S. pastorianus could influence strain phenotypes (in this case, the production of α-acetolactate). Otherwise, there was no functional difference between the gene products as determined by their over-expression with the same promoter. In other cases, functional divergence has been observed for many gene products that influence brewing properties (Iijima and Ogata 2010; Ogata et al. 2013; He et al. 2014). Similar investigations must be carried out with newly created hybrids to determine the level of initial regulatory disorder and also how regulatory issues are resolved over time. Tirosh et al. (2009) measured gene activity in de novo S. cerevisiae × S. paradoxus hybrids and found that both cis- and transregulation could be observed and that this was influenced by the environmental conditions to which the hybrids were exposed. It would be of interest to determine how subgenome activity in new S. cerevisiae × S. eubayanus hybrids is influenced by environmental conditions, particularly those conditions, such as temperature extremes, that have the greatest differential impact on the physiology of the parent strains. Wendland (2014) has suggested that differences in fermentation temperature may have molded the genomes of the Saaz and Frohberg strains, which display differences in their tolerance to low-temperatures (Gibson et al. 2013; Walther et al. 2014). This hypothesis could be tested by adapting cultures of the same hybrid strain in parallel to either high or low temperatures and assessing the genetic changes occurring in each case.
Extensive chromosome loss and intrachromosomal translocations, sequence divergence, and chromosome copy number variation in the genomes of lager yeast (van den Broek et al. 2015) indicate that the S. pastorianus genome is inherently unstable. Such instability is not unexpected given the high level of regulatory incompatibilities (Landry et al. 2007) and functional redundancy that are associated with polyploid hybrids (Kumaran et al. 2013; Selmecki et al. 2015). The lager yeast genome is certainly amenable to change via evolutionary engineering, which has been applied to improve stress tolerance (Blieck et al. 2007; Ekberg et al. 2013; Huuskonen et al. 2010; James et al. 2008) and modify beer flavor profile (Mikkelsen et al. 1979; Strejc et al. 2013). As the possibility of creating artificial lager hybrids has existed for only a short time, we have limited information about the stability or adaptability of newly formed genomes. Previous research has indicated that one subgenome in a laboratory-made hybrid is often more susceptible to change or elimination. This was the case for example with S. cerevisiae × S. uvarum hybrids, where the S. uvarum moiety was gradually reduced after successive meiotic (Antunovics et al. 2005) or mitotic (Masneuf-Pomarède 2007; Sebastiani et al. 2002) divisions. Likewise, Lopandic et al. (2016) noted the loss of S. kudriavzevii chromosomes from an artificial S. cerevisiae × S. kudriavzevii hybrid, particularly after these fertile hybrids underwent meiosis. A similar reduction in S. kudriavzevii DNA has been observed in natural wine and beer hybrids (González et al. 2008; Peris et al. 2012). There is evidence that this differential DNA loss can be due to environmental conditions. Piotrowski et al. (2012) showed that during adaptation to high temperature, there was a progressive loss of S. uvarum chromosomes from a laboratory S. cerevisiae × S. uvarum hybrid. This suggests that parental physiology may direct the evolution of the hybrid genome with, in this example, progressive loss of the cold-tolerant subgenome at high temperature, leaving a greater proportion of the high-temperature-tolerant S. cerevisiae DNA. Whether this can explain the greater contribution of S. eubayanus DNA in the cold-tolerant Saaz lager yeast remains to be seen.
Hybrid generation
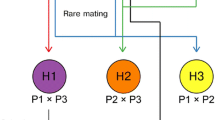
Saccharomyces hybrids can be generated through a variety of methods, including spore-to-spore mating, mass mating, rare mating, and protoplast fusion among others (Figure 2). Here, these methods will be discussed briefly together with an assessment of their advantages and disadvantages. Sexual hybridization occurs when haploid cells of opposite mating type (a or α) meet and fuse (for a recent review on the subject see Merlini et al. (2013)). The traditional approach to yeast breeding is through the mating of cells derived from spores. Spores from the two parent strains can be placed adjacent to one another on an agar plate with the aid of a micromanipulator, i.e., spore-to-spore mating, or randomly mixed together on solid or in liquid growth media, i.e., mass mating. These techniques have been used in the majority of the studies listed in Table 1 (Hebly et al. 2015; Krogerus et al. 2016; Mertens et al. 2015; Sanchez et al. 2012). These approaches have several advantages, including high hybridization frequencies, possible use without selection markers (with spore-to-spore mating), and typically greater genetic stability in the resulting hybrids. However, the parent strains must be able to produce viable spores and physiological traits may be lost or altered through meiotic recombination during spore formation. In the case of mass mating, selection markers (e.g., auxotrophies) or other screening methods are also required to isolate hybrids from the population of parent cells. Steensels et al. (2014) utilized a variant of this approach, where parent strains were first screened for heterothallism (i.e., the spore clones exhibit a stable mating type, and thus do not self-mate) prior to mating. Hybrid status of any isolates can be confirmed through various PCR (e.g., using ITS, interdelta or species-specific primers) or karyotyping techniques (e.g., pulsed-field gel electrophoresis) (Fernández-Espinar et al. 2000; Legras and Karst 2003; Muir et al. 2011).
An overview of different hybridization methods. During a spore-to-spore mating, the diploid (2n) parent strains are first sporulated, after which haploid spores of opposite mating type derived from the two parent strains are brought together and allowed to mate. A diploid (2n) hybrid is formed. During b rare mating, the diploid (2n) parent strains are brought together without any prior sporulation. The cells are not able to directly mate, but rare spontaneous loss of heterozygosity at the mating-type locus can occur in a fraction of the population. As a result, diploid cells with a single mating type, which are able to mate, are formed. A tetraploid (4n) hybrid is formed. During c protoplast fusion, the cell walls of the diploid (2n) parent strains are first digested, after which the protoplasts are brought together and undergo fusion, followed by the regeneration of the cell wall. A tetraploid (4n) hybrid is formed
If either or both of the parent strains one wishes to hybridize are unable to form viable spores, one can apply rare mating. Diploid (or higher ploidy) strains generally have a/α mating type (i.e., a heterozygous mating type locus) and do not directly mate. However, spontaneous loss of heterozygosity at the mating type locus can occur at low frequencies (10−4), resulting in the formation of diploid (or higher) cells with a or α mating types (Hiraoka et al. 2000). These cells may then mate to form polyploid hybrids, which may contain more or less the full genomes of both parent strains. This approach has also been used in many of the studies listed in Table 1 (Choi et al. 2002; Krogerus et al. 2015, 2016; Sato et al. 2002). The most recent of these studies (Krogerus et al. 2016) suggested that higher ploidy lager hybrids produced through rare mating outperformed (in regards to fermentation rate and aroma formation) a diploid lager hybrid formed through spore-to-spore mating. However, as the name implies, these matings occur rarely and the hybridization frequencies are typically low. Furthermore, because of the low mating frequency, selection markers (e.g., auxotrophies) are required to isolate hybrids from the population of parent cells. The genomes of hybrids formed from rare mating also tend to be less stable than those formed from mating of spores (Pérez-Través et al. 2012).
To overcome the disadvantages of low hybridization frequencies and requirement for selection markers, various strategies have been developed. Alexander et al. (2016) describe a technique that can be used to force mating type change in diploid cells by transformation with a plasmid carrying the HO gene under the control of an inducible promoter (this gene is repressed in a/α diploid cells). Expression of HO in a/α diploid cells results in mating type change to a- or α-type, allowing for rare mating with higher hybridization frequencies. The plasmids also carry drug-resistance markers, which allow for the selection of hybrids. Fukuda et al. (2016) describe another approach for selecting diploid cells with either an a or α mating type. In their technique, a/α diploid cells are transformed with a plasmid carrying either the a1 or α2 gene from the mating-type locus together with drug-resistance markers with promoters specific to either the a1 or α2 gene products. When cells containing these plasmids are grown on media containing the particular drug, only cells with an a or α mating type are able to grow (as the a1-α2 dimer will repress the transcription of the drug-resistance gene). Hence, this technique also increased the hybridization frequency of rare mating. However, both these techniques require the transformation of cells with plasmids carrying drug-resistance markers. These plasmids are easily lost from the yeast though, resulting in cells with no exogenous DNA remaining.
The final approach to generating hybrids that will be discussed in this review is protoplast fusion. With this approach, the cell walls of the parent strains are first digested (i.e., protoplasts are formed), after which the cells or protoplasts are brought together and undergo fusion, followed by the regeneration of the cell wall (van Solingen and van der Plaat 1977). As with rare mating, this technique is particularly advantageous for mating strains that rarely form viable spores. Furthermore, protoplast fusion allows for the mating of sexually incompatible cells, e.g., in the formation of intergeneric hybrids (Lucca et al. 2002). Of the studies listed in Table 1, only Mukai et al. (2001) used protoplast fusion. The disadvantages of protoplast fusion are low hybridization frequencies, the need for selection markers, and typically low genome stability in the resulting hybrids. Also, hybrids resulting from protoplast fusion may be considered genetically modified in some regions of the world.
Future prospects and concluding remarks
The interspecific yeast hybrid S. pastorianus already plays a vital role in the modern brewing industry, but its phenotypic potential is limited due to it containing genetic material from only two or three individual yeast strains. To overcome this, the creation of novel brewing yeast hybrids has been shown to be a promising strain development tool for brewing yeast. Hybridization enables the combination and enhancement of a range of phenotypic features from different and diverse parent strains, and the technique has already been used to create yeast hybrids with faster fermentation, more complete sugar use, greater stress tolerance, and more diversified aroma compound production. However, the use of hybridization to improve on several other phenotypic traits still remains unexplored. These include encouraging the formation of antioxidants to increase flavor stability, decreasing the formation of unwanted off-flavors to enhance beer quality, and increasing glycerol formation for better mouthfeel in low-alcohol beer. Furthermore, studies on the use of de novo hybrids for brewing purposes have been mainly limited to hybrids created with S. cerevisiae or S. eubayanus strains as parents. Many other species in the Saccharomyces genus possess traits desirable for brewing, including cold tolerance and high ester formation, and thus represent feasible alternatives to S. eubayanus in interspecific hybrids for lager brewing purposes.
While hybrids possess various enhanced phenotypes in comparison to the parent strains, the molecular mechanisms that control and contribute to the hybrid phenotypes are not fully understood. These phenotypes include the cold and stress tolerance of lager hybrids and heterosis effect observed for aroma formation. With the greater application of sequencing in hybrid studies, it is expected that questions regarding the stability of hybrid genomes, subgenome cooperation and regulation, and the evolutionary history of S. pastorianus will become clearer in the future. There is also the potential for exploiting the inherent instability of hybrid genomes for advanced strain development through adaptive evolution. Hybridization can therefore be explored and utilized further in several ways, yielding powerful and diverse yeast strains for the brewing industry.
References
Aguilera J, Randez-Gil F, Prieto JA (2007) Cold response in Saccharomyces cerevisiae: new functions for old mechanisms. FEMS Microbiol Rev 31:327–341
Alexander W, Peris D, Pfannenstiel B, Opulente D, Kuang M, Hittinger C (2016) Efficient engineering of marker-free synthetic allotetraploids of Saccharomyces. Fungal Genet Biol 89:10–17
Almeida P, Gonçalves C, Teixeira S, Libkind D, Bontrager M, Masneuf-Pomarède I, Allbertin W, Durrens P, Sherman DJ, Marullo P, Hittinger CT, Gonçalves P, Sampaio JP (2014) A Gondwanan imprint on global diversity and domestication of wine and cider yeast Saccharomyces uvarum. Nat Commun 5:4044
Alves S Jr, Herberts R, Hollatz C (2007) Maltose and maltotriose active transport and fermentation by Saccharomyces cerevisiae. J Am Soc Brew Chem 65:99–104
Antunovics Z, Nguyen H-V, Gaillardin C, Sipiczki M (2005) Gradual genome stabilisation by progressive reduction of the Saccharomyces uvarum genome in an interspecific hybrid with Saccharomyces cerevisiae. FEMS Yeast Res 5:1141–1150
Baker E, Wang B, Bellora N, Peris D, Hulfachor A, Koshalek J, Adams M, Libkind D, Hittinger C (2015) The genome sequence of Saccharomyces eubayanus and the domestication of lager-brewing yeasts. Mol Biol Evol 32:2818–2831
Basso R, Alcarde A, Portugal C (2016) Could non-Saccharomyces yeasts contribute on innovative brewing fermentations? Food Res Int 86:112–120
Bellon J, Eglinton J, Siebert T, Pollnitz A, Rose L, de Barros LM, Chambers P (2011) Newly generated interspecific wine yeast hybrids introduce flavour and aroma diversity to wines. Appl Microbiol Biotechnol 91:603–612
Bellon J, Schmid F, Capone D, Dunn B, Chambers P (2013) Introducing a new breed of wine yeast: interspecific hybridisation between a commercial Saccharomyces cerevisiae wine yeast and Saccharomyces mikatae. PLoS One 8:e62053. doi:10.1371/journal.pone.0062053
Bellon J, Yang F, Day M, Inglis D, Chambers P (2015) Designing and creating Saccharomyces interspecific hybrids for improved, industry relevant, phenotypes. Appl Microbiol Biotechnol 99:8597–8609
Bilinski C, Russell I, Stewart G (1986) Analysis of sporulation in brewer’s yeast: induction of tetrad formation. J Inst Brew 92:594–598
Bing J, Han P, Liu W, Wang Q, Bai F (2014) Evidence for a far east Asian origin of lager beer yeast. Curr Biol 24:R380–R381
Bizaj E, Cordente A, Bellon J, Raspor P, Curtin C, Pretorius I (2012) A breeding strategy to harness flavour diversity of Saccharomyces interspecific hybrids and minimize hydrogen sulphide production. FEMS Yeast Res 12:456–465
Blieck L, Toye G, Dumortier F, Verstrepen K, Delvaux F, Thevelein J, Van Dijck P (2007) Isolation and characterization of brewer’s yeast variants with improved fermentation performance under high-gravity conditions. Appl Environ Microbiol 73:815–824
Bolat I, Romagnoli G, Zhu F, Pronk J, Daran J (2013) Functional analysis and transcriptional regulation of two orthologs of ARO10, encoding broad-substrate-specificity 2-oxo-acid decarboxylases, in the brewing yeast Saccharomyces pastorianus CBS1483. FEMS Yeast Res 13:505–517
Caesar R, Palmfeldt J, Gustafsson JS, Pettersson E, Hossein Hashemi S, Blomberg A (2007) Comparative proteomics of industrial lager yeast reveals differential expression of the cerevisiae and non-cerevisiae parts of their genomes. Proteomics 7:4135–4147
Canonico L, Agarbati A, Comitini F, Ciani M (2016) Torulaspora delbrueckii in the brewing process: a new approach to enhance bioflavour and to reduce ethanol content. Food Microbiol 56:45–51
Chen Z (2013) Genomic and epigenetic insights into the molecular bases of heterosis. Nat Rev Genet 14:471–482
Choi B, Jang K, Kim K (2002) Fermentation characteristics of brewing yeast HCS with glucoamylase expression by rare mating and beer analysis. Food Sci Biotechnol 11:34–39
Codón A, Gasent-Ramírez J, Benítez T (1995) Factors which affect the frequency of sporulation and tetrad formation in Saccharomyces cerevisiae baker’s yeast. Appl Environ Microbiol 61:630–638
da Silva T, Albertin W, Dillmann C, Bely M, la Guerche S, Giraud C, Huet S, Sicard D, Masneuf-Pomarède I, de Vienne D, Marullo P (2015) Hybridization within Saccharomyces genus results in homoeostasis and phenotypic novelty in winemaking conditions. PLoS One 10:e0123834. doi:10.1371/journal.pone.0123834
de Barros LM, Bellon J, Shirley N, Ganter P (2002) Evidence for multiple interspecific hybridization in Saccharomyces sensu stricto species. FEMS Yeast Res 1:323–331
Dornbusch H (1997) Prost! The story of German beer. Brewers Publications, Boulder
Dunn B, Sherlock G (2008) Reconstruction of the genome origins and evolution of the hybrid lager yeast Saccharomyces pastorianus. Genome Res 18:1610–1623
Dunn B, Paulish T, Stanbery A, Piotrowski J, Koniges G, Kroll E, Louis E, Liti G, Sherlock G, Rosenzweig F (2013) Recurrent rearrangement during adaptive evolution in an interspecific yeast hybrid suggests a model for rapid introgression. PLoS Genet 9(3):e1003366. doi:10.1371/journal.pgen.1003366
Ekberg J, Rautio J, Mattinen L, Vidgren V, Londesborough J, Gibson B (2013) Adaptive evolution of the lager brewing yeast Saccharomyces pastorianus for improved growth under hyperosmotic conditions and its influence on fermentation performance. FEMS Yeast Res 13:335–349
Fernández-Espinar M, Esteve-Zarzoso B, Querol A, Barrio E (2000) RFLP analysis of the ribosomal internal transcribed spacers and the 5.8S rRNA gene region of the genus Saccharomyces: a fast method for species identification and the differentiation of flor yeasts. Antonie Van Leeuwenhoek 78:87–97
Fu D, Xiao M, Hayward A, Jiang G, Zhu L, Zhou Q, Li J, Zhang M (2015) What is crop heterosis: new insights into an old topic. J Appl Genet 56:1–13
Fukuda N, Kaishima M, Ishii J, Kondo A, Honda S (2016) Continuous crossbreeding of sake yeasts using growth selection systems for a-type and α-type cells. AMB Expr 6:45. doi:10.1186/s13568-016-0216-x
Gallone B, Steensels J, Prahl T, Soriaga L, Saels V, Herrera-Malaver B, Merlevede A, Roncoroni M, Voordeckers K, Miraglia L, Teiling C, Steffy B, Taylor M, Schwartz A, Richardson T, White C, Baele G, Maere S, Verstrepen K (2016) Domestication and divergence of Saccharomyces cerevisiae beer yeasts. Cell 166:1397–1410
Gamero A, Tronchoni J, Querol A, Belloch C (2013) Production of aroma compounds by cryotolerant Saccharomyces species and hybrids at low and moderate fermentation temperatures. J Appl Microbiol 114:1405–1414
García-Ríos E, Querol A, Guillamón JM (2016) iTRAQ-based proteome profiling of Saccharomyces cerevisiae and cryotolerant species Saccharomyces uvarum and Saccharomyces kudriavzevii during low-temperature wine fermentation. J Proteome 146:70–79
Garcia Sanchez R, Solodovnikova N, Wendland J (2012) Breeding of lager yeast with Saccharomyces cerevisiae improves stress resistance and fermentation performance. Yeast 29:343–355
Gayevskiy V, Goddard M (2016) Saccharomyces eubayanus and Saccharomyces arboricola reside in North Island native New Zealand forests. Environ Microbiol 18:1137–1147
Gibson B, Storgårds E, Krogerus K, Vidgren V (2013) Comparative physiology and fermentation performance of Saaz and Frohberg lager yeast strains and the parental species Saccharomyces eubayanus. Yeast 30:255–266
Gibson B, Liti G (2015) Saccharomyces pastorianus: genomic insights inspiring innovation for industry. Yeast 32:17–27
Gibson B, Krogerus K, Ekberg J, Monroux A, Mattinen L, Rautio J, Vidgren V (2015) Variation in α-acetolactate production within the hybrid lager yeast group Saccharomyces pastorianus and the affirmation of the central role of the ILV6 gene. Yeast 32:301–316
Gjermansen C, Sigsgaard P (1981) Construction of a hybrid brewing strain of Saccharomyces carlsbergensis by mating of meiotic segregants. Carlsb Res Commun 46:1–11
Glendinning T (1899) Some practical aspects of the fermentable matter in beer. J Fed Inst Brew 5:20–34
Goddard MR, Greig D (2015) Saccharomyces cerevisiae: a nomadic yeast with no niche? FEMS Yeast Res 15. doi:10.1093/femsyr/fov009
Gonçalves P, Valério E, Correia C, de Almedia J, Sampaio J (2011) Evidence for divergent evolution of growth temperature preference in sympatric Saccharomyces species. PLoS One 6:e20739
Gonçalves M, Pontes A, Almeida P, Barbosa R, Serra M, Libkind D, Hutzler M, Gonçalves P, Sampaio J (2016) Distinct domestication trajectories in top-fermenting beer yeasts and wine yeasts. Curr Biol 26:2750–2761
González S, Barrio E, Gafner J, Querol A (2006) Natural hybrids from Saccharomyces cerevisiae, Saccharomyces bayanus and Saccharomyces kudriavzevii in wine fermentations. FEMS Yeast Res 6:1221–1234
González S, Barrio E, Querol A (2008) Molecular characterization of new natural hybrids of Saccharomyces cerevisiae and S. kudriavzevii in brewing. Appl Environ Microbiol 74:2314–2320
Greig D, Louis E, Borts R, Travisano M (2002) Hybrid speciation in experimental populations of yeast. Science 298:1773–1775
Hammond J, Eckersley K (1984) Fermentation properties of brewing yeast with killer character. J Inst Brew 90:167–177
Hansen J, Cherest H, Kielland-Brandt MC (1994) Two divergent MET10 genes, one from Saccharomyces cerevisiae and one from Saccharomyces carlsbergensis, encode the alpha subunit of sulfite reductase and specify potential binding sites for FAD and NADPH. J Bacteriol 176:6050–6058
He Y, Dong J, Yin H, Chen P, Lin H, Chen L (2014) Monitoring of the production of flavour compounds by analysis of the gene transcription involved in higher alcohol and ester formation by the brewer’s yeast Saccharomyces pastorianus using a multiplex RT-qPCR assay. J Inst Brew 120:119–126
Hebly M, Brickwedde A, Bolat I, Driessen M, de Hulster E, van den Broek M, Pronk J, Geertman J, Daran J, Daran-Lapujade P (2015) S. cerevisiae × S. eubayanus interspecific hybrid, best of both worlds and beyond. FEMS Yeast Res 15. doi:10.1093/femsyr/fov005
Hiraoka M, Watanabe K, Umezu K, Maki H (2000) Spontaneous loss of heterozygosity in diploid Saccharomyces cerevisiae cells. Genetics 156:1531–1548
Horinouchi T, Yoshikawa K, Kawaide R, Furusawa C, Nakao Y, Hirasawa T, Shimizu H (2010) Genome-wide expression analysis of Saccharomyces pastorianus orthologous genes using oligonucleotide microarrays. J Biosci Bioeng 110:602–607
Hornsey I (2003) A history of beer and brewing. Royal Society of Chemistry, Cambridge, p. 720
Hornsey IS (2012) Alcohol and its role in the evolution of human society. RSC Publishing, Cambridge
Huuskonen A, Markkula T, Vidgren V, Lima L, Mulder L, Geurts W, Walsh M, Londesborough J (2010) Selection from industrial lager yeast strains of variants with improved fermentation performance in very-high-gravity worts. Appl Environ Microbiol 76:1563–1573
Iijima K, Ogata T (2010) Construction and evaluation of self-cloning bottom-fermenting yeast with high SSU1 expression. J Appl Microbiol 109:1906–1913
James TC, Usher J, Campbell S, Bond U (2008) Lager yeasts possess dynamic genomes that undergo rearrangements and gene amplification in response to stress. Curr Genet 53:139–152
Janderová B, Cvrcková F, Bendová O (1990) Construction of the dextrin-degrading pof brewing yeast by protoplast fusion. J Basic Microbiol 30:499–505
Johnston J (1965) Breeding yeasts for brewing: II. Production of hybrid strains J Inst Brew 71:135–137
Krogerus K, Gibson B (2013) 125th anniversary review: diacetyl and its control during brewery fermentation. J Inst Brew 119:86–97
Krogerus K, Magalhães F, Vidgren V, Gibson B (2015) New lager yeast strains generated by interspecific hybridization. J Ind Microbiol Biotechnol 42:769–778
Krogerus K, Arvas M, De Chiara M, Magalhães F, Mattinen L, Oja M, Vidgren V, Yue J, Liti G, Gibson B (2016) Ploidy influences the functional attributes of de novo lager yeast hybrids. Appl Microbiol Biotechnol 100:7203–7222
Kumaran R, Yang S-Y, Leu J-Y (2013) Characterization of chromosome stability in diploid, polyploid and hybrid yeast cells. PLoS One 8:e68094
Landaud S, Helinck S, Bonnarme P (2008) Formation of volatile sulfur compounds and metabolism of methionine and other sulfur compounds in fermented food. Appl Microbiol Biotechnol 77:1191–1205
Landry CR, Hartl DL, Ranz JM (2007) Genome clashes in hybrids: insights from gene expression. Heredity 99:483–493
Le Jeune C, Lollier M, Demuyter C, Erny C, Legras J, Aigle M, Masneuf-Pomaréde I (2007) Characterization of natural hybrids of Saccharomyces cerevisiae and Saccharomyces bayanus var. uvarum. FEMS Yeast Res 7:540–549
Legras J, Karst F (2003) Optimisation of interdelta analysis for Saccharomyces cerevisiae strain characterisation. FEMS Microbiol Lett 221:249–255
Legras J, Merdinoglu D, Cornuet J, Karst F (2007) Bread, beer and wine: Saccharomyces cerevisiae diversity reflects human history. Mol Ecol 16:2091–2102
Libkind D, Hittinger C, Valerio E, Gonçalves C, Dover J, Johnston M, Gonçalves P, Sampaio J (2011) Microbe domestication and the identification of the wild genetic stock of lager-brewing yeast. Proc Natl Acad Sci U S A 108:14539–14544
Liti G, Peruffo A, James S, Roberts I, Louis E (2005) Inferences of evolutionary relationships from a population survey of LTR-retrotransposons and telomeric-associated sequences in the Saccharomyces sensu stricto complex. Yeast 22:177–192
Lopandic K, Pfliegler W, Tiefenbrunner W, Gangl H, Sipiczki M, Sterflinger K (2016) Genotypic and phenotypic evolution of yeast interspecies hybrids during high-sugar fermentation. Appl Microbiol Biotechnol 100:6331–6343
Lopes CA, Barrio E, Querol A (2010) Natural hybrids of S. cerevisiae x S. kudriavzevii share alleles with European wild populations of Saccharomyces kudriavzevii. FEMS Yeast Res 10:412–421
López-Malo M, Querol A, Guillamon J (2013) Metabolomic comparison of Saccharomyces cerevisiae and the cryotolerant species S. bayanus var. uvarum and S. kudriavzevii during wine fermentation at low temperature. PLoS One 8:e60135
Lucca M, Spencer J, de Figueroa L (2002) Glycerol and arabitol production by an intergeneric hybrid, PB2, obtained by protoplast fusion between Saccharomyces cerevisiae and Torulaspora delbrueckii. Appl Microbiol Biotechnol 59:472–476
Magalhães F, Vidgren V, Ruohonen L, Gibson B (2016) Maltose and maltotriose utilisation by group I strains of the hybrid lager yeast Saccharomyces pastorianus. FEMS Yeast Res 16. doi:10.1093/femsyr/fow053
Masneuf-Pomarède I, Bely M, Marullo P, Lonvaud-Funel A, Dubourdieu D (2010) Reassessment of phenotypic traits for Saccharomyces bayanus var. uvarum wine yeast strains. Int J Food Microbiol 139:79–86
Masneuf-Pomarède I, Le Jeune C, Durrens P, Lollier M, Aigle M, Dubourdieu D (2007) Molecular typing of wine strains Saccharomyces bayanus var. uvarum using microsatellite markers. Systems Appl Microbiol 30:345–354
Merlini L, Dudin O, Martin S (2013) Mate and fuse: how yeast cells do it. Open Biol 3:130008. doi:10.1098/rsob.130008
Mertens S, Steensels J, Saels V, de Rouck G, Aerts G, Verstrepen K (2015) A large set of newly created interspecific yeast hybrids increases aromatic diversity in lager beers. Appl Environ Microbiol 81:8202–8214
Michel M, Kopecka J, Meier-Dörnberg T, Zarnkow M, Jacob F, Hutzler M (2016) Screening for new brewing yeasts in the non-Saccharomyces sector with Torulaspora delbrueckii as model. Yeast 33:129–144
Mikkelsen JD, Sigsgaard P, Olsen A, Erdal K, Kielland-Brandt MC, Petersen JGL (1979) Thiaisoleucine resistant mutants in Saccharomyces carlsbergensis increase the content of D-amyl alcohol in beer. Carlsb Res Commun 44:219
Minato T, Yoshida S, Ishiguro T, Shimada E, Mizutani S, Kobayashi O, Yoshimoto H (2009) Expression profiling of the bottom fermenting yeast Saccharomyces pastorianus orthologous genes using oligonucleotide microarrays. Yeast 26:147–165
Monerawela C, James T, Wolfe K, Bond U (2015) Loss of lager specific genes and subtelomeric regions define two different Saccharomyces cerevisiae lineages for Saccharomyces pastorianus group I and II strains. FEMS Yeast Res 15. doi:10.1093/femsyr/fou008
Muir A, Harrison E, Wheals A (2011) A multiplex set of species-specific primers for rapid identification of members of the genus Saccharomyces. FEMS Yeast Res 11:552–563
Mukai N, Nishimori C, Fujishige I, Mizuno A, Takahashi T, Sato K (2001) Beer brewing using a fusant between a sake yeast and a brewer’s yeast. J Biosci Bioeng 91:482–486
Mukai N, Masaki K, Fujii T, Iefuhi H (2014) Single nucleotide polymorphisms of PAD1 and FDC1 show a positive relationship with ferulic acid decarboxylation ability among industrial yeasts used in alcoholic beverage production. J Biosci Bioeng 118:50–55
Nakao Y, Kanamori T, Itoh T, Kodama Y, Rainieri S, Nakamura N, Shimonaga T, Hattori M, Ashikari T (2009) Genome sequence of the lager brewing yeast, an interspecies hybrid. DNA Res 16:115–129
Naumov GI, James SA, Naumova E, Louis E, Roberts IN (2000) Three new species in the Saccharomyces sensu stricto complex: Saccharomyces cariocanus, Saccharomyces kudriavzevii, and Saccharomyces mikatae. Int J Syst Evol Microbiol 50:1931–1942
Naumov GI, Nguyen HV, Naumova ES, Michel A, Aigle M, Gaillardin C (2001) Genetic identification of Saccharomyces bayanus var. uvarum, a cider-fermenting yeast. Int J Food Microbiol 65:163–171
Nilsson-Tillgren T, Gjermansen C, Kielland-Brandt M, Petersen J, Holmberg S (1981) Genetic differences between Saccharomyces carlsbergensis and S. cerevisiae. Analysis of chromosome III by single chromosome-transfer. Carlsb Res Commun 46:65–76
Ogata T, Shikata-Myoshi M, Tadami H, Nakazawa N (2011) Isolation of meiotic segregants from a bottom fermenting yeast. J Inst Brew 117:199–205
Ogata T, Kobayashi M, Gibson BR (2013) Pilot scale brewing using self-cloning bottom-fermenting yeast with high SSU1 expression. J Inst Brew 119:17–22
Okuno M, Kajitani R, Ryusui R, Morimoto H, Kodama Y, Itoh T (2016) Next-generation sequencing analysis of lager brewing strains reveals the evolutionary history of interspecies hybridization. DNA Res 23:67–80
Paget C, Schwartz J, Delneri D (2014) Environmental systems biology of cold-tolerant phenotype in Saccharomyces species adapted to grow at different temperatures. Mol Ecol 23:5241–5257
Pedersen MB (1985) DNA sequence polymorphisms in the genus Saccharomyces. II. Analaysis of the gees RDN1, HIS4, LEU2 and Ty transposable elements in Carlsberg, Tuborg, and 22 Bavarian brewing strains. Carlsb Res Commun 50:262–272
Pérez-Través L, Lopes C, Barrio E, Querol A (2012) Evaluation of different genetic procedures for the generation of artificial hybrids in Saccharomyces genus for winemaking. Int J Food Microbiol 156:102–111
Peris D, Belloch C, Lopandić K, Álvarez-Pérez JM, Querol A, Barrio E (2012) The molecular characterization of new types of Saccharomyces cerevisiae x S. kudriavzevii hybrid yeasts unveils a high genetic diversity. Yeast 29:81–91
Peris D, Sylvester K, Libkind D, Gonçalves P, Sampaio J, Alexander W, Hittinger C (2014) Population structure and reticulate evolution of Saccharomyces eubayanus and its lager-brewing hybrids. Mol Ecol 23:2031–2045
Peris D, Langdon Q, Moriarty R, Sylvester K, Bontrager M, Charron G, Leducq J, Landry C, Libkind D, Hittinger C (2016) Complex ancestries of lager-brewing hybrids were shaped by standing variation in the wild yeast Saccharomyces eubayanus. PLoS Genet 12(7):e1006155. doi:10.1371/journal.pgen.1006155
Pfliegler W, Antunovics Z, Sipiczki M (2012) Double sterility barrier between Saccharomyces species and its breakdown in allopolyploid hybrids by chromosome loss. FEMS Yeast Res 12:703–718
Piotrowski J, Nagarajan S, Kroll E, Stanbery A, Chiotti K, Kruckeberg A, Dunn B, Sherlock G, Rosenzweig F (2012) Different selective pressures lead to different genomic outcomes as newly-formed hybrid yeasts evolve. BMC Evol Biol 12:46. doi:10.1186/1471-2148-12-46
Pires E, Teixeira J, Branyik T, Vicente A (2014) Yeast: the soul of beer’s aroma—a review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl Microbiol Biotechnol 98:1937–1949
Plech M, de Visser J, Korona R (2014) Heterosis is prevalent among domesticated but not wild strains of Saccharomyces cerevisiae. G3 4:315–323
Rautio J, Londesborough J (2003) Maltose transport by brewer’s yeasts in brewer’s wort. J Inst Brew 109:251–261
Russell I, Hancock I, Stewart G (1983) Construction of dextrin fermentative yeast strains that do not produce phenolic off-flavours in beer. J Am Soc Brew Chem 41:45–51
Sahara T, Goda T, Ohgiya S (2002) Comprehensive expression analysis of time-dependent genetic responses in yeast cells to low temperature. J Biol Chem 277:50015–50021
Salvadó Z, Arroyo-López FN, Guillamón JM, Salazar G, Querol A, Barrio E (2011) Temperature adaptation markedly determines evolution within the genus Saccharomyces. Appl Environ Microbiol 77:2292–2302
Salvadó Z, Ramos-Alonso L, Tronchoni J, Penacho V, García-Ríos E, Morales P, Gonzalez R, Guillamón JM (2016) Genome-wide identification of genes involved in growth and fermentation activity at low temperature in Sacchaomyces cerevisiae. Int J Food Microbiol 236:38–46
Sampaio JP, Gonçalves P (2008) Natural populations of Saccharomyces kudriavzevii in Portugal are associated with oak bark and are sympatric with S. cerevisiae and S. paradoxus. Appl Environ Microbiol 74:2144–2152
Sato M, Kishimoto M, Watari J, Takashio M (2002) Breeding of brewer’s yeast by hybridization between a top-fermenting yeast Saccharomyces cerevisiae and a cryophilic yeast Saccharomyces bayanus. J Biosci Bioeng 93:509–511
Schade B, Jansen G, Whiteway M, Entian KD, Thomas DY (2004) Cold adaptation in budding yeast. Mol Biol Cell 15:5492–5502
Schnable P, Springer N (2013) Progress toward understanding heterosis in crop plants. Annu Rev Plant Biol 64:71–88
Sebastiani F, Barberio C, Casalone E, Cavalieri D, Polsinelli M (2002) Crosses between Saccharomyces cerevisiae and Saccharomyces bayanus generate fertile hybrids. Res Microbiol 153:53–58
Selmecki A, Maruvka Y, Richmond P, Guillet M, Shoresh N, Sorenson A, De S, Kishony R, Michor F, Dowell R, Pellman D (2015) Polyploidy can drive rapid adaptation in yeast. Nature 519:349–352
Shapira R, Levy T, Shaked S, Fridman E, David L (2014) Extensive heterosis in growth of yeast hybrids is explained by a combination of genetic models. Heredity 113:316–326
Snoek T, Picca Nicolino M, Van den Bremt S, Mertens S, Saels V, Verplaetse A, Steensels J, Verstrepen K (2015) Large-scale robot-assisted genome shuffling yields industrial Saccharomyces cerevisiae yeasts with increased ethanol tolerance. Biotechnol Biofuels 8:32
Spencer J, Spencer D (1977) Hybridization of non-sporulating and weakly sporulating strains of brewer’s and distiller’s yeasts. J Inst Brew 83:287–289
Spencer J, Spencer D, Whittington-Vaughan P, Miller R (1983) Use of mitochondrial mutants in the isolation of hybrids involving industrial yeast strains: IV. Characterization of an intergeneric hybrid, Saccharomyces diastaticus x Hansenula capsulata, obtained by protoplast fusion. Curr Genet 7:159–164
Steensels J, Meersman E, Snoek T, Saels V, Verstrepen K (2014) Large-scale selection and breeding to generate industrial yeasts with superior aroma production. Appl Environ Microbiol 80:6965–6975
Strejc J, Siříštova L, Karabín M, Almeida e Silva JB, Brányik B (2013) Production of alcohol-free beer with elevated amounts of flavouring compounds using lager yeast mutants. J Inst Brew 119:149–155
Stribny J, Querol A, Pérez-Torrado R (2016) Differences in enzymatic properties of the Saccharomyces kudriavzevii and Saccharomyces uvarum alcohol acetyltransferases and their impact on aroma-active compounds production. Front Microbiol 7:00897. doi:10.3389/fmicb.2016.00897
Tai SL, Daran-Lapujade P, Walsh MC, Pronk JT, Daran JM (2007) Acclimation of Saccharomyces cerevisiae to low temperature: a chemostat-based transcriptome analysis. Mol Biol Cell 18(12):5100–5112
Tamai Y, Momma T, Yoshimoto H, Kaneko Y (1998) Co-existence of two types of chromosome in the bottom fermenting yeast, Saccharomyces pastorianus. Yeast 14:923–933
Tirosh I, Reikhav S, Levy A, Barkai N (2009) A yeast hybrid provides insight into the evolution of gene expression regulation. Science 324:659–662
Tubb R, Searle B, Goodey A, Brown A (1981) Rare mating and transformation for construction of novel brewing yeasts. Proc 18th Congr Eur Brew Conv pp. 487–496
Twardowski T, Malyska A (2015) Uninformed and disinformed society and the GMO market. Trends Biotechnol 33:1–3
Urano N, Sahara H, Koshino S (1993) Conversion of a non-flocculent brewer’s yeast to flocculent ones by electrofusion: 1. Identification and characterization of the fusants by pulsed field gel electrophoresis J Biotechnol 28:237–247
Van den Broek M, Bolat I, Nijkamp J, Ramos E, Luttik M, Koopman F, Geertman J, de Ridder D, Pronk J, Daran J (2015) Chromosomal copy number variation in Saccharomyces pastorianus evidence for extensive genome dynamics in industrial lager brewing strains. Appl Environ Microbiol 81:6253–6267
van Solingen P, van der Plaat J (1977) Fusion of yeast spheroplasts. J Bacteriol 130:946–947
Vanderhaegen B, Nevin H, Verachtert H, Derdelinckx G (2006) The chemistry of beer aging—a critical review. Food Chem 95:357–381
Vidgren V, Multanen J, Ruohonen L, Londesborough J (2010) The temperature dependence of maltose transport in ale and lager strains of brewer’s yeast. FEMS Yeast Res 10:402–411
Vidgren V, Viljanen K, Mattinen L, Rautio J, Londesborough J (2014) Three Agt1 transporters from brewer’s yeasts exhibit different temperature dependencies for maltose transport over the range of brewery temperatures (0–20 °C). FEMS Yeast Res 14:601–613
Walther A, Hesselbart A, Wendland J (2014) Genome sequence of Saccharomyces carlsbergensis, the world’s first pure culture lager yeast. G3 4:783–793
Wendland J (2014) Lager yeast comes of age. Eukaryot Cell 13:1256–1265
Winge O (1944) On segregation and mutation in yeast. Compt Rend Trav Lab Carlsberg 24:79–96
Yoshida S, Hashimoto K, Shimada E, Ishiguro T, Minato T, Mizutani S, Yoshimoto H, Tashiro K, Kuhara S, Kobayashi O (2007) Identification of bottom-fermenting yeast genes expressed during lager beer fermentation. Yeast 24:599–606
Acknowledgements
We thank Dr. Arja Laitila for critical reading of the manuscript. Research at VTT was supported by the Alfred Kordelin Foundation, Svenska Kulturfonden - The Swedish Cultural Foundation in Finland, PBL Brewing Laboratory, the Academy of Finland (Academy Projects 276480 and 305453), and the European Union’s Seventh Framework Programme FP7/2007-2013/ under REA grant agreement no. 606795.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Krogerus, K., Magalhães, F., Vidgren, V. et al. Novel brewing yeast hybrids: creation and application. Appl Microbiol Biotechnol 101, 65–78 (2017). https://doi.org/10.1007/s00253-016-8007-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-8007-5