Abstract
The effects of nitrogen sources on growth of sophorolipid-producing yeast, Wickerhamiella domercqiae var. sophorolipid CGMCC 1576 and on production and composition of sophorolipids were studied. Organic nitrogen sources are more favorable for accumulation of biomass than inorganic ones. Presence of ammonium ion from different inorganic nitrogen sources (except NH4HCO3) greatly inhibited the production of lactonic sophorolipids. However, when organic nitrogen sources were used, lactonic sophorolipid production was strongly increased. Production of crystalline lactonic sophorolipids from organic/inorganic nitrogen sources was enhanced with the increase of pH value adjusted by sodium hydroxide or sodium citrate solution. Fourier-transform infrared (FT-IR), gas chromatography mass spectrometry (GC-MS), high-performance liquid chromatography (HPLC), and mass spectra (MS) were employed to compare the composition of sophorolipid mixture obtained from different nitrogen sources. More than 15 acidic sophorolipid molecules and only 4 lactonic sophorolipid molecules were produced by using 1.27 g/l ammonium sulfate as nitrogen source; they were separated by preparative HPLC and their structures were elucidated by MS. These results suggest extraordinary regulatory effects of nitrogen source on growth and sophorolipid synthesis of W. domercqiae var. sophorolipid.
Similar content being viewed by others
Introduction
A selected number of yeast species, such as Candida apicola(Gorin et al. 1961), Candida bogoriensis (Tulloch et al. 1968), Candida bombicola (Spencer et al. 1970), Wickerhamiella domercqiae (Chen et al. 2006a), and Pichia anomala (Thaniyavarn et al. 2008), etc., were reported to produce extracellular biosurfactant as sophorolipids. In recent years, sophorolipids have attracted much attention for their application potential in petroleum, cosmetic, food processing, pharmaceutical, detergent, and environment industries due to their good surface activity, biodegradability, biocompatibility, low toxicity, production process under mild conditions, and production from renewable materials (Lee et al. 2008).
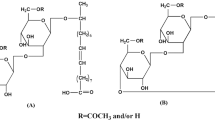
Sophorolipids (SLs) consist of a hydrophilic moiety, one dimeric sugar sophorose molecule, linked by a glycosidic bond to a hydroxyl group on long-chain fatty acid, the lipophilic part (Fig. 1). Sophorolipids produced by yeast fermentation are a mixture composed of up to 20 compounds with similar fundamental structures except for the differences in chain length and saturation degree of fatty acid or in acetylation degree of sophorose and can be classified as two groups, acidic and lactonic SLs (Davila et al. 1993). Different structures of sophorolipid molecules result in differences in their biological and physicochemical properties and can be specifically applied in different fields. Generally, acetylated lactonic sophorolipids were reported to have better antimicrobial and anticancer activities and be used as bacteriostatic agents and anticancer medicine candidates (Hu and Ju 2001; Chen et al. 2006a, b; Shao et al. 2010). In comparison with lactonic sophorolipids, acidic sophorolipids present better foaming capacity and water solubility, which can be much applied in food processing, detergence, oil recovery, heavy metal ion removal, and skin treatment, particularly as agents for fibrinolysis, healing, desquamation, depigmentation, and macrophage activation (Borzeix 1999; Maingault 1999). These considerations prompted the development of different process for specific sophorolipid production.
Some studies regarding the influence of medium composition, pH value, and culture method on sophorolipid production with C. bombicola and C. apicola are available. It was reported that the use of hexadecane as lipophilic substrate resulted in the production of two di-acetylated lactonic sophorolipids with 15-OH or 16-OH palmitic acid (Hu and Ju 2001). Ashby et al. (2006) reported that when fatty acid esters (methyl-soyate, ethyl-soyate, and propyl-soyate), and glycerol were used as fermentation substrates, production of acidic sophorolipids was enhanced. In addition, the sophorolipid molecules obtained from fermentation of C. bombicola on biodiesel co-product stream were primarily in the open-chain acidic form (Ashby et al. 2005). The sophorolipid mixture was also greatly influenced by different pH value of the culture broth. Different pH value resulted in dominant production of either acidic or lactonic sophorolipids. Gobbert et al. (1984) reported that pH 3.5 is optimum for lactonic sophorolipid production by resting cell of Torulopsis bombicola (now known as C. bombicola); Stüwer et al. (1987) demonstrated that cultivation of Torulopsis apicola (now known as C. apicola) at a lower pH value (pH < 2.0) resulted in dominant production of water-soluble acidic sophorolipids and when sodium citrate or sodium hydroxide was added to the culture medium to adjust the pH value to 3.0, C. apicola altered to produce large amounts of crystalline lactonic glycolipids. Furthermore, it was reported that, when using two-stage bioprocesses starting from deproteinized whey concentrate using Cryptococcus curvatus ATCC 20509 and Candida bombicola ATCC 22214, main structures of sophorolipids were neutral lactones (Otto et al. 1999). However, these reports mainly focused on the influences of carbon sources or cultivation method on the production of sophorolipids by C. bombicola and C. apicola. Few studies regarding the influence of different nitrogen (N) sources, inorganic or organic ones, on sophorolipid production can be available. It has been known that sophorolipids was less produced by excess N source in medium. With the increase of the ratio of carbon sources to nitrogen sources (C/N ratio), sophorolipid production by C. apicola was increased obviously (Hommel et al. 1994).
For W. domercqiae, few reports on the effects of medium composition and pH value on sophorolipid production and composition were published. In order to know how nitrogen sources and pH affect biomass, yield, and composition of sophorolipid mixture of W. domercqiae, the influence of different nitrogen sources on sophorolipid production were investigated in the present work.
Materials and methods
Chemicals and reagents
Acetonitrile and methanol were of high-performance liquid chromatography (HPLC) grade and purchased from TEDIA Company Inc (USA). Other chemicals and reagents were of analytical grade and purchased from Tianjin Kaitong Chemical Reagent Co., Ltd and Sinopharm Chemical Reagent Co., Ltd, China.
Microorganism and media
W. domercqiae var. sophorolipid CGMCC 1576 was isolated by our laboratory and preserved in China General Microbiological Culture Collection Center (CGMCC).
The composition of the seed medium was as follows (grams per liter): glucose, 20; yeast extract, 10; and peptone, 20. All fermentation medium contained the following same ingredients (grams per liter): glucose, 80.0; KH2PO4, 1.0; Na2HPO4·12H2O, 1.0; MgSO4·7H2O, 0.5; and oleic acid, 60.0 (v/v) and different organic or inorganic nitrogen sources. Organic nitrogen sources such as yeast extract (total nitrogen content, 9%), peptone (total nitrogen content, 12%), urea, l-glutamic acid, CH3COONH4, and inorganic nitrogen sources as (NH4)2SO4, NH4HCO3, NH4Cl, NH4NO3, and NaNO3 were added to fermentation medium in the same total nitrogen content, respectively.
The strain was cultivated in seed medium (50 ml in a 300-ml flask) in a rotatory shaker at 180 rpm, 30°C for 16 h, then 2% (v/v) of the culture from seed medium was transferred to fermentation medium containing different nitrogen sources (50 ml in a 300-ml flask) and cultivated for 7 days at 200 rpm, 30°C.
Two adjustment methods and three pH controllers (sodium hydroxide, ammonium citrate, and calcium carbonate) were employed to adjust pH value. In one way, pH adjuster was added during the whole fermentation process, and in the other way, adjuster was added at the beginning of cultivation. Fermentation broth was adjusted to pH 3.5 by addition of 1 M sterile sodium hydroxide per 12 h after cultivation for 72 h. When calcium carbonate or ammonium citrate was used, 1 and 5 g/l were added to the fermentation medium at the beginning of the cultivation, respectively.
Determination of cells, residual glucose, and oleic acid
Five milliliters of culture broth was mixed with n-butanol/ethanol/chloroform (10:10:1) of equal volume and then centrifuged at 8,000 rpm for 10 min at room temperature. The solid residue was washed twice with distilled water, and the cell pellet was dried at 50°C to a constant weight.
Residual glucose was measured by bio-sensor SBA-40C (Shandong Academy of Sciences, China). This instrument measures the content of glucose according to the reaction that glucose was oxidized to gluconic acid by immobilized glucose oxidase. Before determination, it is necessary to have a calibration by standard liquids of 50 mg/dl lactic acid–100 mg/dl glutamic acid–glucose (purchased from Shandong Academy of Sciences, China). After the initial sample was diluted to measurable range of 10–150, glucose was determined by bio-sensor SBA-40C with an injection volume of 25 μl. Multiplied by dilution ratio, the concentration of residual glucose was obtained.
Residual oleic acid was determined by extracting with petroleum ether and then determined at OD230 (Zhan 2007). Oleic acid was used as the standard compound, and the concentration of residual oleic acid in the petroleum ether extracts were calculated by standard curve interpolation.
Determination of sophorolipid production
Two volumes of ethyl acetate were added to 0.5 ml fermentation broth, and the lactonic sophorolipid in organic phase was measured by anthrone method (Wodarczak and Buschmann 1995). For the determination of total sophorolipid, 1 ml of acetonitrile was added to 0.5 ml broth to dissolve sophorolipids, and the solution was centrifuged at 10,000 rpm for 10 min; total sugar content in the supernatant was quantified by anthrone method, and residual glucose content was determined by the bio-sensor method mentioned above.
Sophorolipid separation and structure elucidation
The above-mentioned broth/acetonitrile phase were taken out and evaporated under vacuum at 50°C, respectively. The remaining oleic acid was washed with hexane and removed by vacuumed evaporation. Then, the crude sophorolipid was redissolved in 1.0 ml methanol and applied to further analysis by analytical HPLC (SHIMADZU, Japan) with a Venusil MP-C18 column (250 × 4.6 mm, Agela Technologies Inc., USA). Acetonitrile/water was used as the mobile phase at an acetonitrile gradient from 40% to 60% in 15 min followed by an acetonitrile gradient from 60% to 70% in 35 min at a flow rate of 1.0 ml/min. The injection volume was 25 μl, and the eluent was monitored with UV detector at 207 nm. Each separate sophorolipid molecule in total sophorolipid was prepared in quantity by mixing 50 ml broth with 100 ml acetonitrile, followed by using preparative HPLC (SHIMADZU, Japan) with a PrepHT XDB C18 column (250 × 21.2 mm, Agela Technologies Inc., USA). Gradient elution was programmed as the analytical HPLC except for a flow rate of 12 ml/min and an injection volume of 750 μl. Each peak fraction was separately collected, concentrated by vacuumed rotary evaporator, after purity confirmation by analytical reverse phase high-performance liquid chromatography (RP-HPLC) mentioned above, then applied to mass spectra (MS) analysis for structure elucidation on an API4000 mass spectrometer (Applied Biosystems, Foster City, USA), respectively.
Determination of fatty acid moieties of sophorolipid
For the determination of the fatty acid moieties of sophorolipid, total sophorolipids (acidic and lactonic) were cleaved and transesterified in the presence of methanol and H2SO4 to yield the methyl esters of fatty acids following the method described by Davila et al. (1993) with minor modification. Amounts of 50 mg of total sophorolipids were dissolved in 2 ml of 1% H2SO4/methanol solution and 1 ml of toluene as an internal standard. Then, the mixture was heated at 100°C for 1 h. The resulting fatty acid methyl esters were extracted twice with 5 ml of cyclohexane in the presence of 5 ml of 50 g/l NaCl solution. The cyclohexane phase was removed by centrifugation, and the residue was dried. The dried fatty acid esters were dissolved in HPLC grade ethanol and analyzed by GC-MS (SHIMADZU, Japan) equipped with a RTX-5 fused silica column (30 m × 0.32 mm, 0.25 μm coating). Gas chromatography parameters were: N2 carrier; 10:1 (v/v) split; injector at 220°C; column temperature, initially at 190°C for 2 min, then increased by 3°C per min up to 260°C, and then held at 260°C for 5 min; injection volume at 1 μl. MS parameters were: source at 220°C and scan range at 45 to 550 amu (arbitrary mass units) s−1.
Results
Effects of different nitrogen sources on cell growth and sophorolipid production
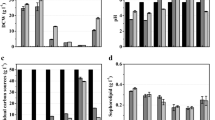
W. domercqiae var. sophorolipid can utilize various nitrogen sources to produce sophorolipid. Yeast extract of 3.0 g/l has been demonstrated to be optimal for lactonic sophorolipid production in our previous work. Therefore, nitrogen contents of different N sources in each medium were all converted to be equivalent to 3.0 g/l yeast extract unless otherwise stated. Yeast growth and sophorolipid production in the media containing different N sources were shown in Table 1.
When organic N sources were added to the medium, higher cell dry weight (CDW) was obtained than using inorganic N sources, with an exception of NH4Cl. Urea and NaNO3 led to maximum and minimum biomass after 7 days cultivation, respectively (Table 1). These results suggested that organic N sources are more suitable for yeast growth.
Glucose was effectively utilized in the early logarithmic growth phase and almost used up at the end of the fermentation for most of the selected N sources except NH4NO3, NH4Cl, l-Glu, and NaNO3. When organic N sources were added to the media, oleic acid was better utilized than that using inorganic ones. Once yeast growth started, pH value of the media dropped immediately in 36 h and almost remained constant for the whole fermentation period. When inorganic N sources, such as (NH4)2SO4, NH4Cl, and NH4NO3 were added, final average pH value was 2.02, lower than that in the case of organic N sources (2.78). Differing from other inorganic N sources, NH4HCO3 could buffer pH value of fermentation broth. NaNO3 was not a suitable N source for both cell growth and sophorolipid production, although with an appropriate pH value (Table 1).
At the time of nitrogen was exhausted, sophorolipid production initiated. Crystalline lactonic sophorolipid production depends strongly on N sources. When the nitrogen content was kept constant, organic N sources were more suitable for lactonic sophorolipid production than inorganic N sources. Ammonium ions from inorganic sources (such as (NH4)2SO4, NH4Cl, and NH4NO3) greatly reduced lactonic sophorolipid production and decreased the proportion of lactonic SL to total SL. When (NH4)2SO4, NH4Cl, NH4NO3, and NaNO3 were used, yields of lactonic sophorolipid produced from consumed glucose (YLac-SL/Glu) were greatly decreased (0.03–0.11 g/g) compared with that using yeast extract, peptone, urea, l-glutamic acid, and CH3COONH4 as N sources (0.16–0.50 g/g); furthermore, much lower ratios of lactonic SL/total SL (4.6–11.9%) from inorganic N sources of (NH4)2SO4, NH4Cl, and NH4NO3 than that from organic N sources (25.8–61.9%) were obtained. The effect of NH4HCO3 was different from other inorganic N sources due to its buffering capacity. The lowest and highest Ylac-SL/Glu were obtained by using (NH4)2SO4/NH4Cl and peptone, respectively. Organic or inorganic N sources showed almost no differences for total sophorolipid production. It can be seen that inorganic N sources as (NH4)2SO4, NH4Cl, NaNO3, or NH4NO3 greatly decreased the production of lactonic sophorolipids while the total sophorolipid production showed similar results as using organic N sources; they were more suitable for acidic sophorolipid production. NH4HCO3 was suitable for lactonic sophorolipid production, which was distinct from other inorganic N sources for its buffering capacity (Table 1).
Effects of ammonium ion on sophorolipid production
Studies of Hommel et al. (1987) demonstrated effects of ammonium sulfate concentration on sophorolipid production by C. apicola; it was reported that sophorolipid production increased with increasing initial ammonium sulfate concentration. In our study, contrary results of sophorolipid production by W. domercqiae were obtained. Production of sophorolipid changed from more lactonic form to more acidic form with the increase of ammonium sulfate concentration. An almost equal amount of total sophorolipid was obtained by yeast extract, ammonium sulfate, or complex N sources of yeast extract and ammonium sulfate when keeping the total N content equal. Increase of the proportion of ammonium sulfate in total N source led to the decrease of pH value and CDW. Furthermore, lactonic sophorolipid production was greatly dropped, especially when 1.27 g/l of ammonium sulfate was used as sole N source; the lactonic sophorolipid production decreased by 61.2% and accounted for only 14.8% of total sophorolipid than that when using yeast extract as sole N source (37.2%). For fermentation with an initial concentration of 3 g/l yeast extract and 4 g/l of ammonium sulfate, the lactonic sophorolipids and total sophorolipids decreased to 4.08 and 29.75 g/1 but with an increase of CDW to 12.90 g/1 (Table 2). This result suggested that excess N content is favor for the growth of W. domercqiae but not for sophorolipid production.
Effect of pH on yeast growth and sophorolipid production
For any N sources, pH of broth was maintained at a constant value after 72 h of cultivation. According to the results of optimal pH experiment with yeast extract as N source, pH 3.5 is optimal for lactonic sophorolipid production (36.55 g/1) by W. domercqiae compared with pH 2.5 (17.05 g/1), 3.0 (30.53 g/1), 4.0 (35.59 g/1), or 4.5 (30.57 g/1) when 1 M NaOH was used to adjust the broth per 12 h after 72 h of cultivation.
For any N sources, adjustment of the pH of culture media by sodium citrate or sodium hydroxide could enhance the growth of yeast cells and production of lactonic sophorolipids (Table 3). Addition of sodium hydroxide to the media is more effective for yeast growth than the addition of sodium citrate and calcium carbonate. By using yeast extract as N source, 70.0% more lactonic sophorolipid production was observed by adjusting the pH with sodium citrate than that without adjustment. Sodium hydroxide solution performed a similar effect with a 57.6% increase of lactonic sophorolipid production. Adjustment of pH could not improve the production of total sophorolipids. When CH3COONH4 was used as N source and sodium hydroxide or calcium carbonate was added to the medium, final pH value of culture broth was 3.87 and 3.31, respectively, higher than 2.71 without adjustment. Addition of sterile sodium hydroxide at a concentration of 1.0 M after cultivation for 72 h per 12 h to adjust the pH to 3.5 resulted in a 16.9% and 13.8% increase of biomass and lactonic sophorolipid. However, no enhancement of total sophorolipid production was observed. Addition of calcium carbonate at concentration of 1.0 g/l at the beginning of the cultivation resulted in a slightly increased biomass, but in an obvious 71.0% decreasing production (7.06 g/l) of crystalline lactonic sophorolipid (Table 3). For (NH4)2SO4, adjustment of pH value up to 3.5 could increase the production of lactonic sophorolipids greatly. Compared with that without pH adjustment, lactonic sophorolipid productions were increased to more than four times in both cases of addition of sodium citrate or sodium hydroxide. Similarly, total sophorolipid production was not improved. When N source is excessive, addition of NaOH had no effect on lactonic sophorolipid production and acidic SL was primarily generated.
For all N sources, when pH was adjusted by sodium citrate or sodium hydroxide addition, the production of lactonic sophorolipid was enhanced, especially when YE or (NH4)2SO4 was used. Calcium carbonate, another pH adjuster, was demonstrated to be helpful for the increase of water-soluble acidic sophorolipid production.
Differences of sophorolipids from yeast extract and ammonium sulfate
Using ammonium sulfate as sole N source resulted in more production of higher polar acidic sophorolipids compared with that by using yeast extract. The sophorolipid mixtures produced by using yeast extract and ammonium sulfate as N sources were characterized by Fourier-transform infrared (FT-IR; Fig. 2). The FT-IR spectra of the mixture generated from yeast extract revealed 3,464 cm−1 (O–H stretch) in its structure. The IR spectra of 2,922 and 2,853 cm−1 revealed the absence of asymmetrical stretching (νs CH2) and symmetrical stretching (νs CH2) of methylene groups, respectively. The C–O absorption band at 1,746 cm−1 may contain the contributions of groups of lactones, esters, or acids. The stretch of C–O band of C (−O)–O–C in lactones exists at 1,164 cm−1, while that from the acetyl esters was found to be at 1,240 cm−1. Furthermore, sugar C–O stretch of C–O–H groups was found to be at 1,097 cm−1, and the band at 1,464 cm−1 corresponded to the C–O–H in plane bending of carboxylic acid (−COOH) in the structure of the product. The IR spectrum revealed the absorption band for C=C at 722 cm-1 (Fig. 2a). These structural details of the crude product were similar to the results in the previous reports (Hu and Ju 2001). Fig. 2b exhibited similar absorption band details as Fig. 2a, but differences of band transmittance of every group indicated the differences of proportion of lactonic or acidic sophorolipids in total sophorolipids obtained from different N sources.
The differences in sophorolipid mixture from yeast extract and ammonium sulfate were also compared by analytical RP-HPLC. As shown in Fig. 3, more than 20 sophorolipid peaks in each sophorolipid mixture were observed by UV detector. Retention time of each peak was similar, but relative peak area had significant differences, which demonstrated that N sources had hardly any influence on the composition of each sophorolipid mixture obtained from different N sources but greatly altered the proportion of each sophorolipid molecules in the mixture. As shown in Fig. 3a, two peaks that occurred at retention time of 27.44 and 32.96 min, accounting for 11.53% and 14.53% of the crude product from yeast extract. However, sophorolipid molecules with higher polarity in the mixture obtained from ammonium sulfate are prominently produced (Fig. 3b). In this case, the two main components occurred at retention time of 27.44 and 32.96 min were decreased to about 1/5 of that obtained from yeast extract. That is, sophorolipid molecules with low polarity were mainly produced from yeast extract, while sophorolipid molecules with high polarity were much more produced from ammonium sulfate. Ammonium ions dramatically inhibited the production of low polar sophorolipids.
Structure elucidation of sophorolipid from ammonium sulfate
Fourteen main peaks with higher area occupancy and sharper absorbance peak were collected and concentrated by vacuum evaporation. The structures of collected sophorolipids were elucidated by MS. Fronting peak (retention time between 1 to 3.8 min) was defined as polar compounds. As can be seen in Fig. 3b and Table 4, the eluted fractions from 1 to 10 were acidic sophorolipids, whereas the fractions from 11 to 14 were lactonic forms. The example mass spectrums of the purified compound SL-10 and SL-14 were presented in Figs. 4 and 5. The mass to charge ratio (m/z) at 724.7 (M + NH4) + and 729.7 (M + Na) + in the spectra revealed that the molecular weight of SL was 706, which is the molecular weight of di-acetylated acidic SL with a C18:1 fatty acid (Fig. 4). In Fig. 5, the mass to charge ratio (m/z) at 689.7 (M + H)+ and 706.8 (M + NH4)+ in the spectra showed that the molecular weight of SL was 688, which is the molecular weight of di-acetylated lactonic SL with a C18:1 fatty acid. The mass difference between the two sophorolipids was 18 amu, consistent with a molecular weight of H2O.
As shown in Table 4, the structures of 19 different sophorolipid molecules differing in acetylation degree, carbon chain length, and unsaturation degree of hydroxyl fatty acid and acidic or lactonic forms were identified. Fifteen sophorolipid molecules were acidic, and only four were lactonic ones, which demonstrated the composition of sophorolipid mixture were greatly influenced by the presence of ammonium sulfate. In the identified sophorolipid molecules, nine were mono-acetylated analogs and seven were di-acetylated sophorolipids. The sophorolipid molecules containing fatty acids with carbon numbers of 16 and 18 were predominant types as previously described (Davila et al. 1993). Components occurred at retention time at 8.331 and 9.205 min were isomers and identified as mono-acetylated acidic SL with a C16:1 fatty acid with acylation at 6′ or 6″ position. Additionally, mono-acetylated acidic SL with a C18:1 fatty acid with a MW at 664 was found at 12.395 and 13.600 min. For SLs with two or without acetyl group, no similar result was obtained. Confirmation of the fatty acid moieties of sophorolipid was obtained by the observed co-elution of methyl esters of fatty acids by GC-MS and compared with the data base. The results indicated the occurrence of fatty acids of C18:2, C18:1, C18:0, C16:2, C16:1, C16:0, and C22:2. The molecular weight (718) of peak 1 brings to an acidic C22:2 sophorolipid, which is inconsistent with the very short retention time, thus, the structure of peak 1 will be further elucidated by NMR and remained to be unidentified component here.
Discussion
There are various factors influencing sophorolipid accumulation, e.g., C/N ratio, nature of precursors, and organic N source additions such as yeast extract, peptone or amino acids, oxygen supply, and temperature (Stüwer et al. 1987). Relatively poor information is available about the influence of organic/inorganic N sources on sophorolipid production and composition of the obtained sophorolipid mixture.
Both growth and sophorolipid synthesis of W. domercqiae var. sophorolipid were strongly affected by different organic or inorganic N source. Organic N sources are more suitable for yeast cell growth and lactonic sophorolipid production. By comparison, cell growth was not good and acidic sophorolipid are mainly generated with inorganic N sources. Hommel et al. (1987) reported that the increase in ammonium ion concentration from ammonium sulfate can increase crystalline sophorolipid synthesis of C. bombicola. On the contrary, the increase in ammonium sulfate concentration in media inhibited lactonic sophorolipid formation for W. domercqiae var. sophorolipid.
For growing cells of C. bombicola, the pH range between 3.5 and 4.5 was found to be suitable for sophorolipid formation, and the pH optimum was around pH 3.5 for the resting cell of C. bombicola (Spencer et al. 1967; Gobbert et al. 1984). Similar to C. apicola, adjustment of pH by NaOH or C6H5O7Na3 showed an increase in lactonic sophorolipid production by W. domercqia with different N sources (Stüwer et al. 1987). However, addition of CaCO3 could not increase crystalline sophorolipid production, although pH value increased. Our results suggested that both N sources and pH are factors involved in the complex regulation of sophorolipid accumulation. The regulation mechanism of the sophorolipid production at genomic and transcriptomic levels is underway in our laboratory.
References
Ashby RD, Nunez A, Solaiman DKY, Foglia TA (2005) Sophorolipid biosynthesis from a biodiesel co-product stream. J Am Oil Chem Soc 82:625–630
Ashby RD, Solaiman DKY, Foglia TA (2006) The use of fatty acid esters to enhance free acid sophorolipid synthesis. Biotechnol Lett 28:253–260
Borzeix CF (1999) Use of sophorolipids comprising diacetyl lactones as agent for stimulating skin fibroblast metabolism. World patent 99/62479
Chen J, Song X, Zhang H, Qu YB (2006a) Production, structure elucidation and anticancer properties of sophorolipid from Wickerhamiella domercqiae. Enzyme Microb Technol 39:501–506
Chen J, Song X, Zhang H, Qu YB, Miao JY (2006b) Sophorolipid produced from the new yeast strain Wickerhamiella domercqiae induces apoptosis in H7402 human liver cancer cells. Appl Microbiol Biotechnol 72:52–59
Davila AM, Marchal R, Monin N, Vandecasteele JP (1993) Identification and determination of individual sophorolipids in fermentation products by gradient elution high-performance liquid chromatography with evaporative light-scattering detection. J Chromatogr 648:139–149
Gobbert U, Lang S, Wagner F (1984) Sophorose lipid formation by resting cells of Torulopsis bombicola. Biotechnol Lett 6:225–230
Gorin PAJ, Spencer JFT, Tulloch AP (1961) Hydroxy fatty acid glycosides of sophorose from Torulopsis magnoliae. Can J Chem 39:846–855
Hommel R, Stüwer O, Stuber W, Haferburg D, Kleber HP (1987) Production of water-soluble surface-active exolipids by Torulopsis apicola. Appl Microbiol Biotechnol 26:199–205
Hommel RK, Weber L, Weiss A, Himmelreich U, Rilke O, Kleber HP (1994) Production of sophorose lipid by Candida (Torulopsis) apicola grown on glucose. J Biotechnol 33:147–155
Hu YM, Ju LK (2001) Purification of lactonic sophorolipids by crystallization. J Biotechnol 87:263–272
Lee YJ, Choi JK, Kim EK, Youn SH, Yang EJ (2008) Field experiments on mitigation of harmful algal blooms using a sophorolipid–yellow clay mixture and effects on marine plankton. Harmful Algae 7:154–162
Maingault M (1999) Utilization of sophorolipids as therapeutically active substances or cosmetic products, in particular for the treatment of the skin. US Patent 5,981,497
Otto RT, Daniel HJ, Pekin G, Muller-Decker K, Furstenberger G, Reuss M, Syldatk C (1999) Production of sophorolipids from whey II: product composition, surface active properties, cytotoxicity and stability against hydrolases by enzymatic treatment. Appl Microbiol Biotechnol 52:495–501
Shao LJ, Song X, Ma XJ, Li H, Qu YB (2010) Bioactivities of sophorolipid with different structures against human esophageal cancer cells. J Surg Res. doi:https://doi.org/10.1016/j.jss.2010.09.013
Spencer JFT, Tulloch AP, Gorin PAJ (1967) Oil glycosides of sophorose and fatty acid esters thereof. US Patent 3 312 684
Spencer JFT, Gorin PAJ, Tulloch AP (1970) Torulopsis bombicola sp.n. Antonie Leeuwenhoek 36:129–133
Stüwer O, Hommel R, Haferburg D, Kleber HP (1987) Production of crystalline surface-active glycolipids by a strain of Torulopsis apicola. J Biotechnol 6:259–269
Thaniyavarn J, Chianguthai T, Sangvanich P, Roongsawang N, Washio K, Morikawa M, Thaniyavarn S (2008) Production of sophorolipid biosurfactant by Pichia anomala. Biosci Biotech Bioch 72:2061–2068
Tulloch AP, Spencer JFT, Deinema MH (1968) A new hydroxy fatty acid sophoroside from Candida bogoriensis. Can J Chem 46:345–348
Wodarczak S, Buschmann N (1995) Analytical methods for alkylpolyglucosides. GIT Lab Fachz 5:410–411
Zhan HY (2007) Measuring oil content in waste water using UV-spectrum. J Gansu Lianhe Univ Nat Sci 21:65–66
Acknowledgment
This study was funded by Encouragement Foundation of Shandong Province for Young Scientists (2006BS02010), Key Scientific and Technological project of Shandong Province (2007GG10002002), National Natural Science Foundation of China (no. 30870048 and no. 30970052), and National Key Technology R&D Program in the 11th Five-Year Plan of China (no. 2008BAI63B08).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, Xj., Li, H., Shao, Lj. et al. Effects of nitrogen sources on production and composition of sophorolipids by Wickerhamiella domercqiae var. sophorolipid CGMCC 1576. Appl Microbiol Biotechnol 91, 1623–1632 (2011). https://doi.org/10.1007/s00253-011-3327-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3327-y