Abstract
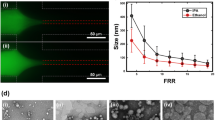
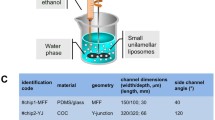
Liposomes are spherical vesicles enclosed by phospholipid bilayers. Nanoscale liposomes are widely employed for drug delivery in the pharmaceutical industry. In this study, nanoscale liposomes are fabricated using the microfluidic hydrodynamic focusing (MHF) approach, and the effects of flow rate ratio (FRR) on liposome size and drug loading efficiency are studied. Fluorescein isothiocyanate modified dextran is used as a hydrophilic drug simulant and Nile red is used as a hydrophobic drug simulant. The experiment results show that hydrophilic drug simulant loading efficiency increases as FRR increases and eventually plateaues at around 90% loading efficiency. The hydrophobic drug simulant loading efficiency and FRR have a positive linear correlation when FRR varies from 10 to 50. Concurrent loading of both hydrophilic and hydrophobic drug simulants maintains the same loading efficiencies as those of loading each drug simulant alone. A negative correlation between liposome size and FRR is also confirmed. Unloaded liposomes and hydrophilic drug-loaded liposomes are of the same sizes, and are smaller than the ones loaded with the hydrophobic drug simulants alone or combined. The results suggest tunable liposome size and drug loading efficiency with the MHF technique. This provides evidence to encourage further studies of microfluidic liposome fabrication in the pharmaceutical industry.







Similar content being viewed by others
References
Bangham AD, Standish MM, Watkins JC (1965) Diffusion of univalent ions across the lamellae of swollen phospholipids. Am J Mol Biol 13(1):238
Barenholz Y (2003) Relevancy of drug loading to liposomal formulation therapeutic efficacy. J Liposome Res 13(1):1
Batzri S, Korn ED (1973) Single bilayer liposomes prepared without sonication. Biochim Biophys Acta Biomembr 298(4):1015
Budai M, Szogyi M (2001) Liposomes as drug carrier systems. Preparation, classification and therapeutic advantages of liposomes. Acta Pharm Hung 71(1):114
Carugo D, Bottaro E, Owen J, Stride E, Nastruzzi C (2016) Liposome production by microfluidics: potential and limiting factors. Sci Rep 6:25876
Gregoriadis G (1984) Liposome technology: volume III: targeted drug delivery and biological interaction. CRC Press, Boca Raton, FL
Gregoriadis G, Leathwood PD, Ryman BE (1971) Enzyme entrapment in liposomes. FEBS Lett 14(2):95
Güven A, Ortiz M, Constanti M, O’Sullivan CK (2009) Rapid and efficient method for the size separation of homogeneous fluorescein-encapsulating liposomes. J Liposome Res 19(2):148
Hoffman JR, Tasciotti E, Molinaro R (2018) Microfluidic assembly of liposomes with tunable size and coloading capabilities. In: Multiple myeloma. Humana Press, New York, NY, pp 205–214
Jahn A, Vreeland WN, DeVoe DL, Locascio LE, Gaitan M (2007) Microfluidic directed formation of liposomes of controlled size. Langmuir 23(11):6289
Litzinger DC, Buiting AM, van Rooijen N, Huang L (1994) Effect of liposome size on the circulation time and intraorgan distribution of amphipathic poly (ethylene glycol)-containing liposomes. Biochim Biophys Acta Biomembr 1190(1):99
Liu DZ, Chen WY, Tasi LM, Yang SP (2000) Microcalorimetric and shear studies on the effects of cholesterol on the physical stability of lipid vesicles. Coll Surf A 172(1):57
Maeki M, Kimura N, Sato Y, Harashima H, Tokeshi M (2018) Advances in microfluidics for lipid nanoparticles and extracellular vesicles and applications in drug delivery systems. Adv Drug Deliv Rev 128:84
Massing U, Fuxius S (2000) Liposomal formulations of anticancer drugs: selectivity and effectiveness. Drug Resist Updates 3(3):171
Maulucci G, De Spirito M, Arcovito G, Boffi F, Castellano AC, Briganti G (2005) Particle size distribution in DMPC vesicles solutions undergoing different sonication times. Biophys J 88(5):3545
Pidgeon C, McNeely S, Schmidt T, Johnson JE (1987) Multilayered vesicles prepared by reverse-phase evaporation: liposome structure and optimum solute entrapment. Biochem 26(1):17
Szoka F, Papahadjopoulos D (1978) Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci USA 75(9):4194
Tan YC, Hettiarachchi K, Siu M, Pan YR, Lee AP (2006) Controlled microfluidic encapsulation of cells, proteins, and microbeads in lipid vesicles. J Am Chem Soc 128(17):5656
Torchilin VP (2005) Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov 4(2):145
Yang X, Zhao X, Phelps MA, Piao L, Rozewski DM, Liu Q, Lee LJ, Marcucci G, Grever MR, Byrd JC, Dalton JT (2009) A novel liposomal formulation of flavopiridol. Int J Pharm 365(1):170
Zook JM, Vreeland WN (2010) Effects of temperature, acyl chain length, and flow-rate ratio on liposome formation and size in a microfluidic hydrodynamic focusing device. Soft Matter 6(6):1352
Acknowledgements
The authors thank Lu Wang for her valuable intellectual discussions. W-Z.S. Lin was funded by the USC Office of the Provost. All dynamic light scattering results were obtained using equipment provided by the USC NanoBioPhysics Core Facility. Soft lithography was done in the USC Keck Photonics Clean Room under the USC Center for Photonic Technology. Ultracentrifugation was processed using the equipment provided by the USC Roberts Research Lab. This work was supported in part by NIH grant R21-CA204708.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, WZ.S., Malmstadt, N. Liposome production and concurrent loading of drug simulants by microfluidic hydrodynamic focusing. Eur Biophys J 48, 549–558 (2019). https://doi.org/10.1007/s00249-019-01383-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-019-01383-2